Gaseous propane (C 3 H 8 ) is burned in a steady-flow, constant-pressure process at 100 kPa
Question:
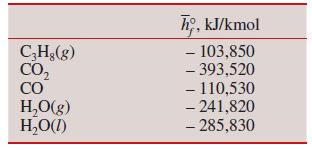
Gaseous propane (C3H8) is burned in a steady-flow, constant-pressure process at 100 kPa with 200 percent theoretical air. During the combustion process, 90 percent of the carbon in the fuel is converted to CO2 and 10 percent is converted to CO. Determine
(a) The balanced combustion equation
(b) The dew-point temperature of the products, in °C
(c) The heat transfer from the combustion chamber, in kJ, after 100 kmol of fuel are burned when the reactants enter the combustion chamber at 25°C and the products are cooled to 25°C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:





