One means of producing liquid oxygen from atmospheric air is to take advantage of the phase-equilibrium properties
Question:
One means of producing liquid oxygen from atmospheric air is to take advantage of the phase-equilibrium properties of oxygen–nitrogen mixtures. This system is illustrated in Fig. P16–122. In this cascaded-reactors system, dry atmospheric air is cooled in the first reactor until liquid is formed. According to the phase-equilibrium properties, this liquid will
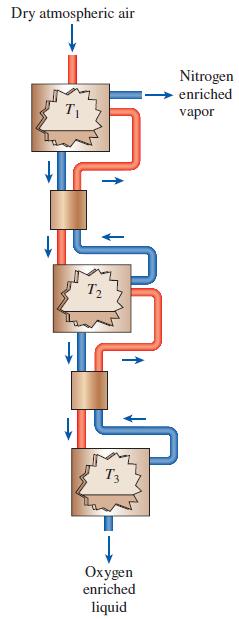
be richer in oxygen than in the vapor phase. The vapor in the first reactor is discarded while the oxygen-enriched liquid leaves the first reactor and is heated in a heat exchanger until it is again a vapor. The vapor mixture enters the second reactor where it is again cooled until a liquid that is further enriched in oxygen is formed. The vapor from the second reactor is routed back to the first reactor while the liquid is routed to another heat exchanger and another reactor to repeat the process once again. The liquid formed in the third reactor is very rich in oxygen. If all three reactors are operated at 1 atm pressure, select the three temperatures that produce the greatest amount of 99 percent pure oxygen.
Step by Step Answer:

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu





