A container contains a substance in gaseous and liquid phases at room temperature (Fig. 6.16). The container
Question:
A container contains a substance in gaseous and liquid phases at room temperature (Fig. 6.16). The container is closed by a piston of surface area A, held back by a spring of elastic constant k. We neglect the mass of the piston. For simplicity, we neglect the volume of the liquid compared to that of the gas. The atmospheric pressure is p0 and assumed independent of temperature.
a) Determine the temperature derivative of the gas pressure![]()
when there is no liquid phase present in the container.
b) Determine the temperature derivative of the gas pressure![]()
when liquid is present.
Figure 6.16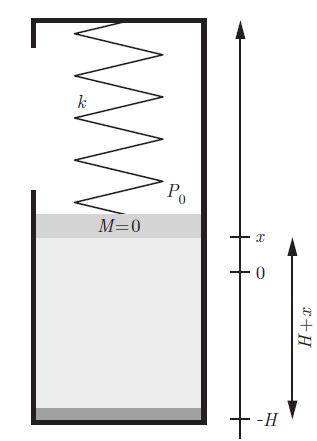
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:





