A liquid is at equilibrium with its vapour. The vapour is assumed to be an ideal gas.
Question:
A liquid is at equilibrium with its vapour. The vapour is assumed to be an ideal gas. The liquid has a molar latent heat of vaporisation ℓℓg that depends on temperature, with ℓℓg = A − BT, where A and B are constants. Apply the Clausius–Clapeyron relation (6.50) and consider that the molar volume of the liquid phase is negligible compared to the vapour phase, i.e. vℓ ≪ vg. Use the ideal gas law (5.47) for the vapour phase. Show that at equilibrium at a temperature T, the vapour pressure p depends on temperature according to Dupré’s law,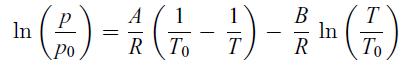
where p0 is the vapour pressure at T0.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:





