An ideal gas characterised by the coefficient c found in relation (5.62) and the coefficient =
Question:
An ideal gas characterised by the coefficient c found in relation (5.62) and the coefficient γ = (c + 1) /c undergoes a Rankine engine cycle consisting of four reversible processes:• 1−→ 2 : isobaric expansion• 2−→ 3 : adiabatic expansion• 3−→ 4 : isobaric compression• 4−→ 1 : adiabatic compressionThus, the cycle is represented by a rectangle in a (T, S) diagram (Fig. 7.25). Analyse this cycle by using the following instructions:a) Draw the (p, V) diagram of a Rankine cycle for an ideal gas.b) Determine the works performed W12, W23, W34 and W41 and the work performed per cycle W in terms of the enthalpies H1, H2, H3 and H4.c) Find the heat provided by the hot reservoir Q+ = Q12 in terms of the enthalpies H1, H2, H3 and H4.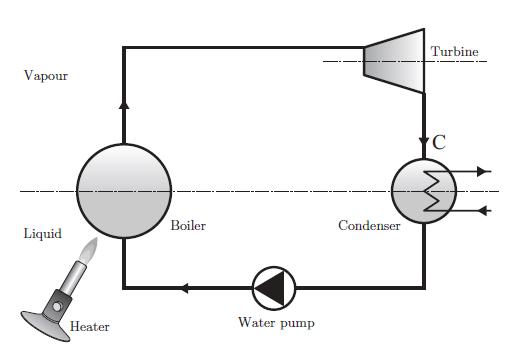
Diagram of the Rankine engine for a biphasic fluid.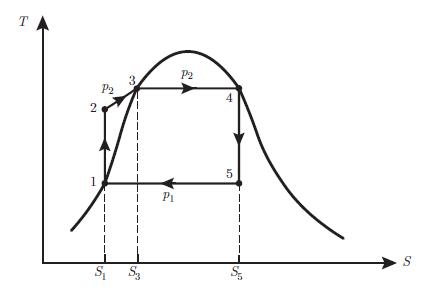
(T, S) diagram of the Rankine cycle for a biphasic fluid.d) Determine the efficiency of the Rankine cycle for an ideal fluid defined as,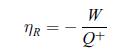
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet




