Assume that a binary mixture and its components can be modeled using the van der Waals equation
Question:
Assume that a binary mixture and its components can be modeled using the van der Waals equation of state, both for the liquid and the vapor phase. Assume also that the mixture equation of state parameters can be calculated using so-called van der Waals one-fluid mixture rules,
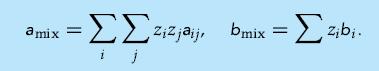
(a) Derive the fugacity coefficient for component i in the mixture and show that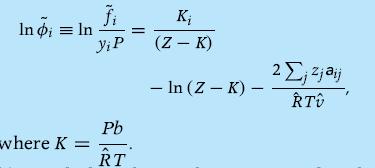
(b) Similarly, obtain the equation for the activity coefficient of component i, γi.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





