Question:
Carbon dioxide at P1?and T1 is contained inside a piston?cylinder. It undergoes polytropic compression according to the process equation PVn = constant.?
a. Derive the formula for the energy transfer as work done by the piston to compress CO2 to a state with P2 > P1. ?he CO2 in the initial state is at a pressure of 10 bar, at a temperature of 280 K, and it occupies a volume of 0.001 m3. The polytropic exponent of this compression process has been determined and is n = 1.4. The final pressure reached by CO2 is 20 bar. Using the tables in Appendix A.9 for data, calculate:
b. The mass of CO2 inside the cylinder,
c. The specific volume of the fluid in the initial and final states,d. The distance traveled by the piston,e. The temperature of the fluid at the final state,f. The work done by the piston,g. The energy transfer as heat and its direction.
Data From Appendix A.9
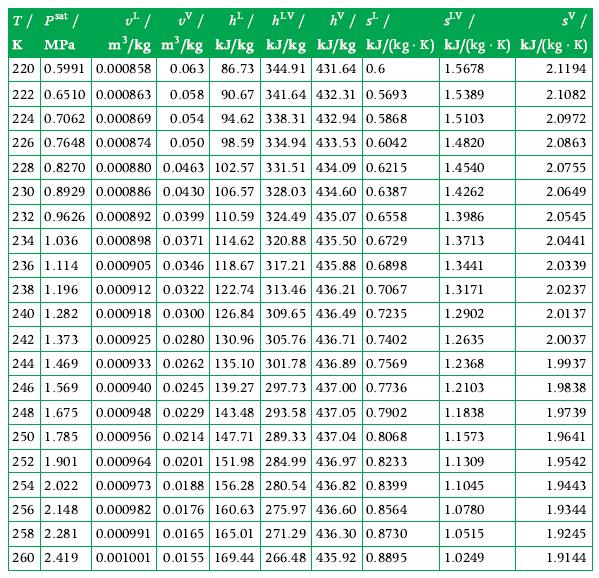
Transcribed Image Text:
T/ psat /
к MPa
220 0.5991 0.000858 0.063 86.73 344.91 431.64 0.6
v² / UV /h²/h²V / h / s² /
SLV /
m³/kg m³/kg kJ/kg kJ/kg kJ/kg kJ/(kg K) kJ/(kg K) kJ/(kg .K)
.
1.5678
2.1194
1.5389
1.5103
1.4820
222 0.6510 0.000863 0.058 90.67 341.64 432.31 0.5693
224 0.7062 0.000869 0.054 94.62 338.31 432.94 0.5868
226 0.7648 0.000874 0.050 98.59 334.94 433.53 0.6042
228 0.8270 0.000880 0.0463 102.57 331.51 434.09 0.6215
230 0.8929 0.000886 0.0430 106.57 328.03 434.60 0.6387
232 0.9626 0.000892 0.0399 110.59 324.49 435.07 0.6558
234 1.036 0.000898 0.0371 114.62 320.88 435.50 0.6729
236 1.114 0.000905 0.0346 118.67 317.21 435.88 0.6898
238 1.196 0.000912 0.0322 122.74 313.46 436.21 0.7067
240 1.282 0.000918 0.0300 126.84 309.65 436.49 0.7235
242 1.373 0.000925 0.0280 130.96 305.76 436.71 0.7402
244 1.469 0.000933 0.0262 135.10 301.78 436.89 0.7569
246 1.569 0.000940 0.0245 139.27 297.73 437.00 0.7736
248 1.675 0.000948 0.0229 143.48 293.58 437.05 0.7902
250 1.785 0.000956 0.0214 147.71 289.33 437.04 0.8068
252 1.901 0.000964 0.0201 151.98 284.99 436.97 0.8233
0.000973 0.0188 156.28 280.54 436.82 0.8399
160.63 275.97 436.60 0.8564
254 2.022
256 2.148
0.000982 0.0176
0.000991 0.0165
165.01 271.29 436.30 0.8730
258 2.281
260 2.419 0.001001 0.0155 169.44 266.48 435.92 0.8895
1.4540
1.4262
1.3986
1.3713
1.3441
1.3171
1.2902
1.2635
1.2368
1.2103
1.1838
1.1573
1.1309
1.1045
1.0780
1.0515
1.0249
2.1082
2.0972
2.0863
2.0755
2.0649
2.0545
2.0441
2.0339
2.0237
2.0137
2.0037
1.9937
1.9838
1.9739
1.9641
1.9542
1.9443
1.9344
1.9245
1.9144







