Estimate E cell for the half reaction. 2H 2 O + 2e - H 2 +
Question:
Estimate E°cell for the half reaction. 2H2O + 2e- → H2 + 2OH- given the following values of ΔGof :
H2O(l) = –237 kJ/ mol H2(g) = 0.0 OH-(aq) = –157 kJ/ mol e- = 0.0 Compare this value of E°cell with the value of E°cell given in Table.
Table
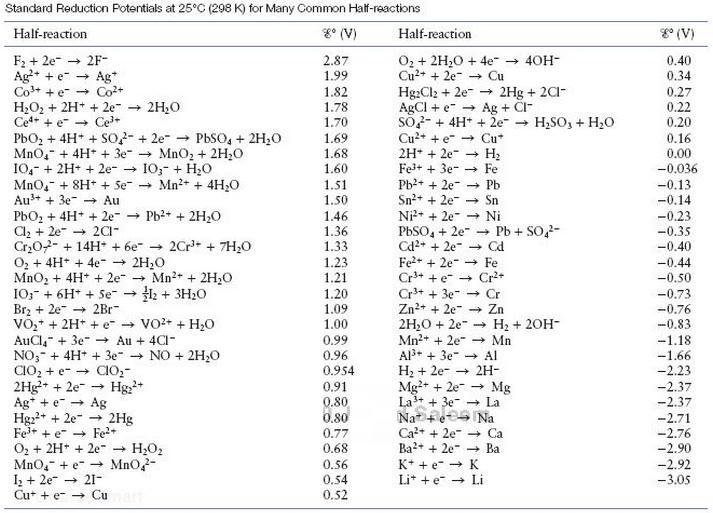
Transcribed Image Text:
Standard Reduction Potentials at 25°C (298 K) for Many Common Half-reactions g° (V) g° (V) Half-reaction Half-reaction O2 + 2H,0 + 4e - 40H- Cu2+ + 2e- - Cu Hg:Cl2 + 2e- AGCI + e- → Ag + Cl- So- + 4H* + 2e- → H,SO, + H20 Cu2+ + e- → Cu* 2H* + 2e Fel+ + 3e- Pb2+ + 2e- Sn2+ + 2e- Ni2* + 2e- → Ni PBSO, + 2e- - Pb + SO2- Cd2+ + 2e- Fe+ + 2e" Cr3+ + e Cr+ Cr+ + 3e- - Cr Zn2+ + 2e Zn 2H;0 + 2e- Mn2+ + 2e" - Mn Al3+ + 3e Al H2 + 2e- Mg+ + 2e → Mg La3+ + 3e- La F2 + 2e- 2F- Ag+ + e Ag* Co+ + e Co+ H2O2 + 2H* + 2e" 2H20 Ce+ + e - Ce+ PbO, + 4H* + SO2- + 2e- - PbSO, + 2H20 Mno,- + 4H* + 3e- → MnO, + 2H;0 10,- + 2H+ + 2e- I0;- + H20 MnO,- + 8H* + Se + Mn2+ + 4H;0 Au3+ + 3e- - Au PbOz + 4H* + 2e- - Pb?+ + 2H20 Cl, + 2e- Cr,02- + 14H + 6e- - O2 + 4H+ + 4e- + 2H,0 MnO, + 4H+ + 2e IO;- + 6H* + Se → H2 + 3H20 Brz + 2e- + 2Br- VO,* + 2H* + e VO2+ + H,0 AuCl, + 3e" - Au + 4CI- NO;- + 4H* + 3e- - NO + 2H20 CIO, + e- → CIO,- 2Hg+ + 2e-- Ag* + e + Ag Hgz2+ + 2e- - 2Hg Fe* + e - Fe+ O, + 2H* + 2e" - H2O2 MnO,- + e- → Mno,- I+ 2e → 21- Cu* + e- → Cu 2.87 0.40 0.34 1.99 - 2Hg + 2C1- 1.82 1.78 1.70 1.69 1.68 1.60 0.27 0.22 0.20 0.16 0.00 -0.036 Н Fe -0.13 -0.14 1.51 1.50 Pb Sn -0.23 1.46 1.36 1.33 1.23 + 201- -0.35 -0.40 2Cr* + 7H,0 - Cd Fe -0.44 Mn2+ + 2H;0 -0.50 1.21 1.20 1.09 1.00 -0.73 -0,76 H2 + 20H- -0.83 0.99 0.96 0.954 -1.18 -1.66 -2.23 + 2H- Hg;+ 0.91 -2.37 -2.37 0.80 0.80 Na Na -2.71 -2.76 -2.90 Ca2+ + 2e- - Ca Ba?+ + 2e" 0.77 0.68 - Ba 0.56 0,54 0.52 K* + e- → K -2.92 Lit +e → Li -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
The two values agree to two significant ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Estimate E cell for the half reaction. 2H 2 O + 2e - H 2 + 2OH - given the following values of G o f : H 2 O(l) = 237 kJ/ mol H 2 (g) = 0.0 OH - (aq) = 157 kJ/ mol e - = 0.0 Compare this value of E...
-
Given the following values of x, s, and n, form a 90% confidence interval for 2. a. x = 21, s = 2.5, n = 50 b. x = 1.3, s = .02, n = 15 c. x = 167, s = 31.6, n = 22 d. x = 9.4, s = 1.5, n = 5
-
Write an equation for the half reaction in which a potassium atom, K, is oxidized.
-
Independent Challenge 1 You are the operations manager for the Chicago Arts Alliance. Each year the group revisits the number and types of activities they support to better manage their budgets. For...
-
Ms. Lynch has a choice of two assets: The first is a risk-free asset that offers a rate of return of rf, and the second is a risky asset (a china shop that caters to large mammals) that has an...
-
If we use a 0.05 significance level in analysis of variance with the sample data given in Exercise 1, what is the P-value? What should we conclude? If a passenger abhors late flight arrivals, can...
-
A mass hanging from a spring undergoes vertical simple harmonic motion. a. Where in the motion is the magnitude of the net force equal to zero? b. Where in the motion is the velocity equal to zero?...
-
A chemically reacting mixture is stored in a thin-walled spherical container of radius r l = 200 mm, and the exothermic reaction generates heat at a uniform, but temperature-dependent volumetric rate...
-
About Patagonia: What is the role of the digital channels of the brand Patagonia under investigation in creating a relevant user experience? Do they provide a great user experience to your opinion?...
-
Study the scenario and complete the questions that follow: Mango Airlines Mango Airlines operates flights within the South African local market. It has flights from Johannesburg to Cape Town,...
-
When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is oxidized and that hydrogen is reduced, write the balanced equation for the reaction. How many...
-
Glucose is the major fuel for most living cells. The oxidative breakdown of glucose by our body to produce energy is called respiration. The reaction for the complete combustion of glucose is C 6 H...
-
What are the characteristics of an online newsroom?
-
What factors besides intelligence could influence a person's performance on an intelligence test?
-
How does Aristotle define the genres of tragedy and comedy? What characteristics set them apart?
-
What does john hiddle mean by his statement that you can't cage a person;a person isn't a bird?
-
Variable pay may also be called a. relational returns Ob.pensions c. stock options Od. incentives
-
An engineer is building a web page and creates an external style sheet to store their CSS declarations. In order to reference this style sheet from their HTML code, the engineer needs to add a ( n )...
-
An accounting period is based on the balance sheet. Agree or disagree and defend your position.
-
Subtract the polynomials. (-x+x-5) - (x-x + 5)
-
Use bond energies (Table 13.6) to show that the pre-ferred products for the decomposition of N2O3 are NO2 and NO rather than O2 and N2O. (The NO single-bond energy is 201 kJ/ mol.) (Hint: Consider...
-
Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g) 2NH3(g)...
-
Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g) 2NH3(g)...
-
explain the solution: ACCT 223 1) * Anderson Corporation had the following information for the month ended September 30, 2023: The balance per the company's general leder at 9/30 was $100,000. The...
-
Code needs to be changed to pass test cases provided. Students will apply concepts of advanced Backtracking. Your solution for each test case must run within 0.31 seconds. Otherwise, no credit will...
-
An abstract class is ____________. 1: a class with all overriding methods 2:a class that has nothing but pure virtual methods 3:a class with all virtual methods 4:a class that has at least one pure...

Study smarter with the SolutionInn App


