The wave function for the hydrogen-atom quantum state represented by the dot plot shown in figure, which
Question:
The wave function for the hydrogen-atom quantum state represented by the dot plot shown in figure, which has n = 2 and ? = m? = 0, is in which a is the Bohr radius and the subscript on ?(r) gives the values of the quantum numbers n, ?, m?.(a) Plot ?2200(r) and show that your plot is consistent with the dot plot of figure.(b) Show analytically that ?2200(r) has a maximum at r = 4a.(c) Find the radial probability density P200(r) for this state.(d) Show that and thus that the expression above for the wave function ?200(r) *zo6(r) has been properly normalized.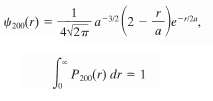
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Probability & Statistics For Engineers & Scientists
ISBN: 9780130415295
7th Edition
Authors: Ronald E. Walpole, Raymond H. Myers, Sharon L. Myers, Keying
Question Posted:





