Vapor-liquid equilibrium data at 101.3 kPa are given for the chloroform-methanol system. From these data, prepare plots
Question:
Vapor-liquid equilibrium data at 101.3 kPa are given for the chloroform-methanol system. From these data, prepare plots like Figures 4.6b and 4.6c. From the plots, determine the azeotropic composition and temperature at 101.3 kPa. Is the azeotrope of the minimum- or maximum-boilingtype?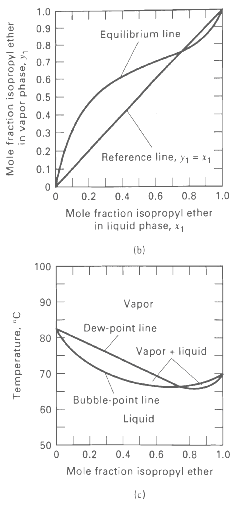
Transcribed Image Text:
1.0 0.9 Equilibrium line 0.8 0.7E 0.6E 0.5 0.4 0.3 0.2 Reference line, y1 = 11 0.1 F 0.6 0.8 1.0 0.2 0.4 Mole fraction isopropyl ether in liquid phase, x1 (b) 100 90 Vapor Dew-point line 80 Vapor • liquid 70 Bubble-point line 50 Liquid 50 0.2 0.4 0.6 0.8 1.0 Mole fraction isopropyl ether Mole fraction isopropyl ether in vapor phase, y, Temperature, "C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
See plots below From these plots a minimumboiling azeo...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The following equations are given for the liquid-phase activity coefficients of the water (W)-acetic acid (A) system. Find the dew point and bubble point of a mixture of composition xw = 0.5, xA =...
-
Frequency tables are given for the first 100 digits in the decimal representation of 77 and the first 100 digits in the decimal representation of 22/7. a. Construct histograms representing the...
-
Liquid water at 200 kPa and 15C is heated in a chamber by mixing it with superheated steam at 200 kPa and 150C. Liquid water enters the mixing chamber at a rate of 4.3 kg/s, and the chamber is...
-
Google, the immensely popular Web search engine, has been touted as the closest thing the Web has to an ultimate answer machine. Although this is debatable, of course, it is far more difficult to...
-
An aging analysis of Reiko Limited's accounts receivable at December 31, 2012 and 2011 showed the following: 1. At December 31, 2011, the unadjusted balance in Allowance for Doubtful Accounts was a...
-
Mulholland Corp., a lessee, entered into a non-cancellable lease agreement with Galt Manufacturing Ltd., a lessor, to lease special-purpose equipment for a period of seven years. Mulholland follows...
-
Britfly describe the meaning of the terms (a) safeguarding of assets, (b) reliability of the financial records, and (c) transactions as these terms relate to internal accounting control.
-
Hoban Company uses special journals and a general journal. The following transactions occurred during September 2012. Sept. 2 Sold merchandise on account to H. Wash, invoice no. 101, $720, terms...
-
Consider the two pucks shown in the figure. As they move towards each other, the momentum of each puck is equal in magnitude and opposite in direction. Given that Vi, green = 11.0 m/s, and m blue is...
-
Maury bought one full bitcoin in March 2021. His basis in the coin is $6,118. In July of 2022, he donated 0.2 of the coin to a qualified charitable organization. The fair market value of a full coin...
-
Using vapor pressure data from Exercises 4.6 and 4.8 and the enthalpy data provided below: (a) Construct an h-x-y diagram for the benzene-toluene system at 1 atm (101.3 kPa) based on the use of...
-
Vapor-liquid equilibrium data at 101.3 kPa are given for the water-formic acid system. From these data, prepare plots like Figures 4.7b and 4.7c. From the plots, determine the azeotropic composition...
-
What do you think are common irrelevant details that college students place on rsums?
-
Without mentioning payment, Mary accepts the services of Razi, a contractor, and is pleased with the work. Is there a contract between them? a. Yes, there is an express contract. b. Yes, there is an...
-
Show that decimation is a time-varying, but periodically time-invariant, operation and that interpolation is a time-invariant operation.
-
Joe England sold equipment on May 10,2017 for \(\$ 100,000\). He bought the equipment on November 7, 2015 for \(\$ 140,000\), and accumulated depreciation at the date of sale was \(\$ 60,000\). Joe...
-
Equity classifications for nongovernment not-for-profit hospitals include all of the following except a. invested in capital assets, net of related debt. b. unrestricted net assets. c. permanently...
-
The sequence \[x=0.125,0.25,0.5,1,2,4\] is filtered by a filter with transfer function \[H(z)=\frac{1}{3}\left(1+z^{-1}+z^{-2} ight)\] and the result is decimated by 2 . The output is then upsampled...
-
Due to crossing over within an inversion loop, a heterozygote with a pericentric inversion may produce gametes that carry a. a deletion. b. a duplication. c. a translocation. d. both a deletion and a...
-
Explain how the graph of each function can be obtained from the graph of y = 1/x or y = 1/x 2 . Then graph f and give the (a) Domain (b) Range. Determine the largest open intervals of the domain over...
-
Discuss various investment roles played by ETFs.
-
A vaporliquid mixture at 250F and 500 psia contains N 2 , H 2 S, CO 2 , and all the normal paraffins from methane to heptane. Use Figure 2.4 to estimate the K-value of each component. Which...
-
One thousand kmol/h of rich gas at 70F with 25% C 1 , 15% C 2 , 25% C 3 , 20% nC 4 , and 15% nC 5 by moles is to be absorbed by 500 kmol/h of nC 10 at 90F in an absorber at 4 atm. Calculate by the...
-
When calculating multicomponent distillation, why is it best to list the components in order of decreasing volatility? In such a list, do the two key components have to be adjacent?
-
9- Assume that printer interrupt priority: 2, disk interrupt priority:4, serial line interrupt priority: 6, Ethernet interrupt priority: 5. Determine the order of interrupt services according to: A...
-
4- Assume that printer interrupt priority: 2, disk interrupt priority:4, Ethernet interrupt priority: 5. A user program starts at t = 0 and takes 40 seconds to complete. At t = 10, a disk interrupt...
-
Robbie is a 3 year old boy in the bur oak room at little.ly early learning centre. You have been one of robbies educators for the past two weeks. You and your colleagues have been concerned about...

Study smarter with the SolutionInn App


