One hundred pound-moles per hour of a mixture of 60 mol% methanol in water at 30oC and
Question:
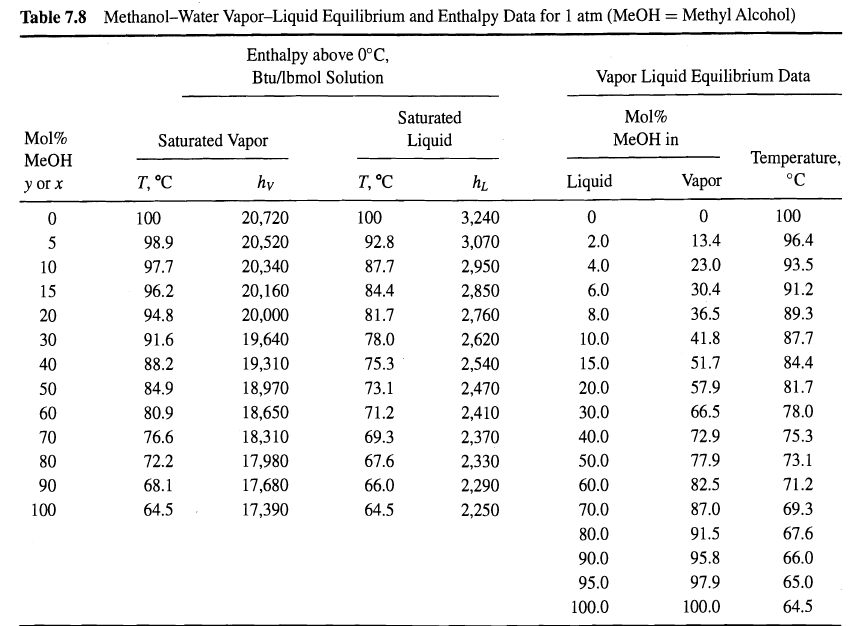
One hundred pound-moles per hour of a mixture of 60 mol% methanol in water at 30oC and 1 atm is to be separated by distillation at the same pressure into a liquid distillate containing 98 mol% methanol and a bottoms liquid product containing 96 mol% water. Enthalpy and equilibrium data for the mixture at 1 atm are given in Table 7.8. The enthalpy of the feed mixture is 765 Btu/Ibmol.
(a) Using the given data, plot an enthalpy'concentration diagram.
(b) Devise a procedure to determine, from the diagram of part (a), the minimum number of equilibrium stages for the condition of total reflux and the required separation.
(c) From the procedure developed in part (b), determine N. Why is the value independent of the feed condition?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





