Diffusion in a gas is the random motion of particles involved in the net movement of a
Question:
Diffusion in a gas is the random motion of particles involved in the net movement of a substance from an area of high concentration to an area of low concentration. The process of diffusion also takes place in solution. Medical scientists are interested in the rate of diffusion of pharmaceutical compounds through tumours. They can model the factors that affect the rate of diffusion of these drugs by conducting investigations using coloured compounds (to model the drugs) and gelatin, a jelly-like substance (to model the tumours). The kinetic energy of particles depends on their mass and the speed they travel at. So at a given temperature, large particles will travel slower on average than smaller particles. Imagine that you are a member of a research team trying to find out how the rate of diffusion through gelatin depends on the relative molecular mass (Mr) of a drug.
a. i. Predict how you think that the rate of diffusion will be affected as the relative molecular mass of a drug increases. Explain your reasoning.
ii. Display your prediction on a sketch graph, including any relevant units.
b. In the experiment you are about to plan, identify the:
i. The independent variable
ii. The dependent variable.
c. The research team can make thin discs of gelatin to cover the central area of Petri dishes, leaving space around the edge of each disc to place a solution of the coloured dyes under investigation:
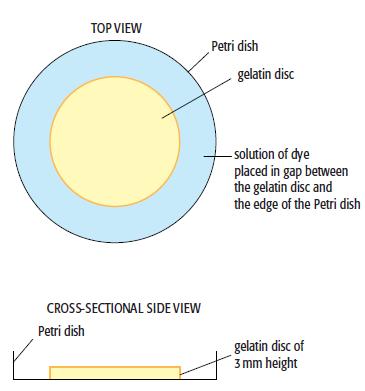
You have been given five coloured powders of dyes, labelled A to E, to test. These have relative molecular masses of 486, 534, 686, 792 and 886, respectively. You are also provided with a stopclock/watch and a ruler with a millimetre scale. You can also use any other common laboratory apparatus needed to complete the investigation. The diffusion is a slow process and the team carry out some trial runs to get a rough idea how quickly the dyes diffuse through the gelatin. They decide to monitor the experiment for 72 hours. Describe how you would carry out the experiment, making sure that you include how to:
■ Ensure the same number of dye molecules is used in each test
■ Ensure that the Petri dish is kept under the same conditions throughout all of the experiments
■ Measure the rate of diffusion
■ Produce reliable results.
d. Two of the dyes are classified as ‘harmful’ and are hazardous if absorbed through the skin or are inhaled. State any precautions you would take to minimise the risk.
e. Draw a table with headings that show clearly the data you would record when carrying out your experiments and any values you would need to calculate in order to construct a graph to check your prediction in part a. The headings must include the appropriate units. Ensure that the table covers all the detail relating to the five dyes listed in part c.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





