a. A starting point for modeling the thermodynamics of polymers in solution is to use the Flory-Huggins
Question:
a. A starting point for modeling the thermodynamics of polymers in solution is to use the Flory-Huggins model with the Flory χ parameter assumed to be a constant. For mixtures of polystyrene in toluene, in which VPS = 1000VT, χ = 0.6 is a reasonable estimate of the value for that parameter. Plot the activity coefficients of polystyrene and toluene as a function of the mole fraction of toluene at 298 K, assuming that the molecular weight of toluene is 92 and that of the polystyrene in this solution is 90 000.
b. Plot these activity coefficients versus the toluene volume fraction, and also plot the activity coefficients as a function of toluene mass fraction.
c. Usually, to accurately fit experimental data, the χ parameter cannot be taken to be a constant, but must be a function of both temperature and composition. One proposed model is
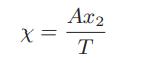
where T is the absolute temperature in kelvins, x2 is the mole fraction of polymer, and A is a constant. Using this expression, derive the equations for the activity coefficients and for the excess enthalpy of mixing Hex of polystyrene-toluene solutions as a function of toluene mole fraction.
d. If the value of the A parameter in the above equation is 1500 K, plot the activity coefficients of polystyrene and toluene as a function of the mole fraction, mass fraction, and volume fraction of toluene at 298 K.
e. Plot the heat of mixing for polystyrene-toluene mixtures as a function of the mole fraction, mass fraction, and volume fraction of toluene at 298 K.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





