The activity of a substance, which is a function of temperature, pressure and composition, is defined as
Question:
The activity of a substance, which is a function of temperature, pressure and composition, is defined as follows:
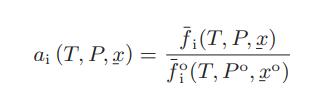
where f̅oi (T,Po, xo) is the standard-state fugacity of species i at the standard-state pressure Po and standard-state composition xo (which could be the pure component state or one of the various Henry’s law standard states).
a. Using this definition of the activity, prove for a binary mixture at constant temperature and pressure that
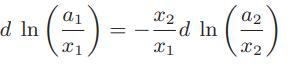
b. For fixed standard-state temperature, derive an expression for how the species activity changes with temperature at constant pressure and composition.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





