Assuming that the van der Waals equation of state, is satisfied by two pure fluids and by
Question:
Assuming that the van der Waals equation of state,
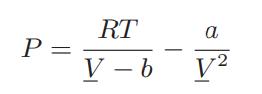
is satisfied by two pure fluids and by their mixture, and that the van der Waals one-fluid rules
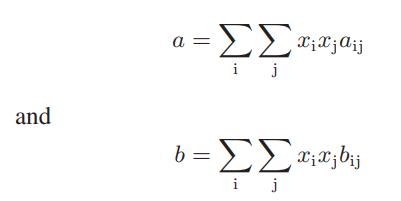 with bij = bji apply to the mixture, derive expressions for
with bij = bji apply to the mixture, derive expressions for
a. The excess volume change on mixing at constant T and P
b. The excess enthalpy and internal energy changes on mixing at constant T and P
c. The excess entropy change on mixing at constant T and P
d. The excess Helmholtz and Gibbs energy changes on mixing at constant T and P
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





