From experimental data it is known that at moderate pressures the volumetric equation of state may be
Question:
From experimental data it is known that at moderate pressures the volumetric equation of state may be written as
![]()
where the virial coefficient B is a function of temperature only. Data for nitrogen are given in the table.
a. Identify the Boyle temperature (the temperature at which B = 0) and the inversion temperature [the temperature at which μ = (∂T/∂P)H = 0] for gaseous nitrogen.
b. Show, from the data in the table, that at temperatures above the inversion temperature the gas temperature increases in a Joule-Thomson
expansion, whereas it decreases if the initial temperature is below the inversion temperature.
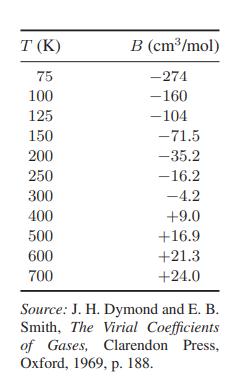
c. Describe how you would find the inversion temperature as a function of pressure for nitrogen using Fig. 3.3-3 and for methane using Fig. 3.3-2.
Fig. 3.3-2.
Fig. 3.3-3
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





