The Clausius equation of state is a. Show that for this volumetric equation of state b. For
Question:
The Clausius equation of state is
![]()
a. Show that for this volumetric equation of state
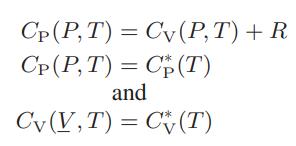
b. For a certain process the pressure of a gas must be reduced from an initial pressure P1 to the final pressure P2. The gas obeys the Clausius equation of state, and the pressure reduction is to be accomplished either by passing the gas through a flow constriction, such as a pressure-reducing valve, or by passing it through a small gas turbine (which we can assume to be both reversible and adiabatic). Obtain expressions for the final gas temperature in each of these cases in terms of the initial state and the properties of the gas.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





