In some cases if pure liquid A and pure liquid B are mixed at constant temperature and
Question:
In some cases if pure liquid A and pure liquid B are mixed at constant temperature and pressure, two liquid phases are formed at equilibrium, one rich in species A and the other in species B. We have proved that the equilibrium state at constant T and P is a state of minimum Gibbs energy, and the Gibbs energy of a twophase mixture is the sum of the number of moles times the molar Gibbs energy for each phase. What would the molar Gibbs free energy versus mole fraction curve look like for this system if we could prevent phase separation from occurring? Identify the equilibrium compositions of the two phases on this diagram. The limit of stability of a single phase at constant temperature and pressure can be found from d2G = 0 or
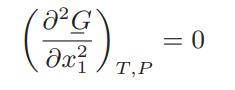 Identify the limits of single-phase stability on the Gibbs energy versus mole fraction curve.
Identify the limits of single-phase stability on the Gibbs energy versus mole fraction curve.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





