Redo Problem 13.10 using Aspen Plus. Problem 13.10 Hydrogen gas can be produced by the following reactions
Question:
Redo Problem 13.10 using Aspen Plus.
Problem 13.10
Hydrogen gas can be produced by the following reactions between propane and steam in the presence of a nickel catalyst:
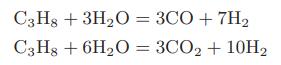
a. Compute the standard heat of reaction and the standard-state Gibbs energy change on reaction for each of the reactions at 1000 and 1100 K.
b. What is the equilibrium composition of the product gas at 1000 K and 1 bar if the inlet to the catalytic reactor is pure propane and steam in a 1-to-10 ratio?
c. Repeat calculation (b) at 1100 K
Transcribed Image Text:
C3H8 + 3H₂O = 3CO + 7H₂ C3H8 + 6H₂O = 3CO₂ + 10H₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
here is the solution to Problem 1310 using Aspen Plus a Standard heat of reaction and standardstate Gibbs energy change The standard heat of reaction ...View the full answer

Answered By

Nicole omwa
Being a highly skilled tutor with at least 5 years of tutoring experience in different areas, I learned how to help diverse learners in writing drafts of research papers, actual research papers and locate credible sources. My assurance is built upon my varied knowledge of a variety of subjects. Furthermore, my involvement and interaction with numerous learners of all levels has allowed me to understand my clients' specific demands. Ultimately, this has aided me in being a better coach to learners to better their grades. Essentially, my responsibilities as a tutor would include:
Teaching abilities that assist pupils in enhancing their academic performance
Personal interaction with learners to make them understand abstract concepts
Inducing new skills and knowledge into their academic journeys
Fostering individual reflection, and independent and critical thinking
Editing and proofreading
Because I am constantly available to respond to your queries, you may decide to rely on me whenever you require my assistance. As an assurance, my knowledge skills and expertise enable me to quickly assist learners with different academic challenges in areas with difficulty in understanding. Ultimately, I believe that I am a reliable tutor concerned about my learner's needs and interests to solve their urgent projects. My purpose is always to assist them in comprehending abstract schoolwork and mastering their subjects. I also understand that plagiarism is a severe offense and has serious ramifications. Owing to this, I always make it a point to educate learners on the numerous strategies to have uniquely unique solutions. I am familiar with the following formatting styles:
MLA
APA
Harvard
Chicago
IEEE
Communication is always the key in every interaction with my learners. Hence, I provide timely communication about the progress of assigned projects. As a result, I make sure that I maintain excellent communication with all of my clients. I can engage with all of my customers more effectively, assisting them with their unique academic demands. Furthermore, I attempt to establish a solid working relationship with my leaners I have exceptional abilities in the below areas;
Sociology
History
Nursing
Psychology
Literature
Health and Medicine
Chemistry
Biology
Management
Marketing
Business
Earth Science
Environmental Studies
Education
Being a teacher who aces in diverse fields, I provide various academic tasks, which include;
Academic Reports
Movie Reviews
Literature Reviews
Annotated bibliographies
Lab reports
Discussion posts
Dissertations
Case study analyses
Research proposals
Argumentative Essays
I guarantee you high-quality Papers!!!!!
5.00+
17+ Reviews
32+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Write and balance the chemical reaction of carbon monoxide forming solid carbon and carbon dioxide vapor. Determine the equilibrium constant at 700 K and 750 K. Will solid carbon form at the...
-
Hydrogen gas can be produced by the following reactions between propane and steam in the presence of a nickel catalyst: Neglecting any other competing reactions: (a) Compute the equilibrium constants...
-
Synthesis gas can be produced by the catalytic re-forming of methane with steam. The reactions are: CH 4 (g) + H 2 O(g) CO(g) + 3H 2 g) CO(g) + H 2 O(g) CO 2 (g) + H 2 (g) Assume equilibrium is...
-
Find an equation in cylindrical coordinates for the equation given in rectangular coordinates. y = x
-
Friendly Freddies is an independently owned major appliance and electronics discount chain with seven stores in a Midwest metropolitan area. Rapid expansion has created the need for careful planning...
-
Show that, for an adiabatic atmosphere, p = C()k, where C is constant, that p/po = [1(k - 1)gz / kRT o ] k/(k-1) where k = cp/cv Compare this formula for air at 5 km altitude with the U.S. standard...
-
Gibbs' free energy of a pure fluid approaches at constant temperature (a) Infinity (b) Minus infinity (c) Zero (d) None of these.
-
Josh Smith has compiled some of his personal financial data in order to determine his liquidity position. The data are as follows. Account Amount Cash ............ $3,200 Marketable securities .........
-
An object rolls off a tabletop with a horizontal velocity v2,0 = 4 m/s. The table is at a height yo = 1.05 m, above the floor. Use a coordinate system with its origin on the floor directly beneath...
-
Redo Problem 13.5 using Aspen Plus. Problem 13.5 The production of NO by the direct oxidation of nitrogen, occurs naturally in internal combustion engines. This reaction is also used to commercially...
-
Redo Problem 13.11 using Aspen Plus. Problem 13.11 An important step in the manufacture of sulfuric acid is the gas-phase oxidation reaction Compute the equilibrium conversion of sulfur dioxide to...
-
Each of the following items must be considered in preparing a statement of cash flows (indirect method) for Bastille Inc., which follows IFRS, for the year ended December 31, 2020. 1. Equipment that...
-
Below are the instructions on how I need to form this research essay. My research topic deals with the personal journey, experiences, and motivation behind choosing a career in pediatric nursing. It...
-
How do negotiation frameworks designed for international conflict resolution adapt to address the nuances of cultural differences, and what are the key factors that contribute to their success or...
-
What specific mediation techniques have been found most effective in resolving deep-seated conflicts within culturally diverse teams, and how do these techniques influence the overarching power...
-
Heidi is preparing her tax return for the year and is looking at ways to save on her tax bill. Heidi worked full time in the day and tended bar at night. Her daytime employer reported her income for...
-
Thus, if I were a jail official attempting to strike a balance between the prisoner's freedom of speech and the prison security concerns that arise from Arias' numerous prison interviews, I would...
-
How do management reports produced by a public accounting firms engagement management system for the current year engagement assist them in bidding on the job in subsequent years? In justifying to...
-
Global.asax is used for: a. declare application variables O b. all other answers are wrong O c. declare global variables O d. handle application events
-
If the pressure in the tire on your car is 32.0 lbf/in 2 (or psi), what is its pressure in SI units? Assume that on the surface of the Earth, g = 9.81 m/s 2 = 32.2 ft/s 2 (i.e., each to three...
-
Suppose the mass in Example 2.1 was 50.0 slugs. What would be its weight in lbf (pounds force)? Assume that on the surface of the Earth, g = 9.81 m/s 2 = 32.2 ft/s 2 (i.e., each to three significant...
-
What would the 5.00 slug mass in Example 2.1 weigh on the Moon where the acceleration of gravity is only 1/6 of that on Earth? Assume that on the surface of the Earth, g = 9.81 m/s 2 = 32.2 ft/s 2...
-
A bond has 10 years to maturity, a coupon rate of 5%(coupons paid semi-annually), a yield to maturity of 6%, and a face value of 1000. Calculate the bonds (Macaulay) duration. Enter your answer as an...
-
What is the acceleration of mass m on the inclined plane? Take positive acceleration to be up the ramp.
-
A three year dual currency bond that pays annual coupons of 50 NZD and a principal value of 1000 AUD at maturity has a present value of NZD 1250. The one, two and three year yield to maturity on...

Study smarter with the SolutionInn App


