A reaction was believed to occur by the following mechanism. (a) Write the overall reaction. (b) Write
Question:
A reaction was believed to occur by the following mechanism.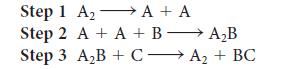
(a) Write the overall reaction.
(b) Write the rate law for each step and indicate its molecularity.
(c) What are the reaction intermediates?
(d) A catalyst is a substance that accelerates the rate of a reaction and is regenerated in the process. Which substance is functioning as a catalyst in the reaction?
Transcribed Image Text:
Step 1 A₂ A + A → Step 2 A + A + B A₂B Step 3 A₂B + CA₂+ BC
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a The overall reaction can be written as A2 2B C 2A BC b The rate law for each s...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The following mechanism has been proposed for the gasphase reaction between HBr and NO 2 : (a) Write the overall reaction. (b) Write the rate law for each step and indicate its molecularity. (c) What...
-
The reaction I2(aq) + OCl2(aq) IO2(aq) + Cl2(aq) is believed to occur by the following mechanism: Write the rate law for this reaction.
-
The isomerization of cyclopropane, C3H6, is believed to occur by the mechanism shown in the following equations: Here C3H6* is an excited cyclopropane molecule. At low pressure, Step 1 is much slower...
-
Consider a random walk consisting of equi-probable p = q = 1/2 steps in left or right directions. However the step length at ith step is given by e-, i = 1,2,3..... N, with > 0 a constant. Calculate...
-
Contemporary computers often have more than 100MB of physical memory. Suppose the page size is 2KB. How many entries would an associative memory need in order to implement a page table for the memory?
-
Picasso Company is a wholesale distributor of professional equipment and supplies. The companys sales have averaged about $900,000 annually for the 3-year period 20122014. The firms total assets at...
-
Water is flowing in a pipe of diameter \(20 \mathrm{~cm}\) with a pressure gradient of \(3000 \mathrm{~Pa} / \mathrm{m} . \mu=\) \(0.001 \mathrm{~Pa} \cdot \mathrm{s}\). Find the wall shear stress....
-
The list that follows presents Shah Companys accounts (in alphabetical order) as of March 31, 2014. The list does not include the amount of Accounts Payable. Prepare a trial balance with the proper...
-
Segmentation is key to identifying the right target markets. As a start-up company, SOCIAL LITE Vodka knew it had to be extremely focused on segmentation. Its alcoholic beverages were made with 100...
-
Substance A decomposes in a first-order reaction and its half life is 355 s. How much time must elapse for the concentration of A to decrease to (a) One-eighth of its initial concentration; (b)...
-
The decomposition of gaseous dinitrogen pentoxide in the reaction 2 N 2 O 5 (g) 4 NO 2 (g) + O 2 (g) gives the data shown here at 298 K. (a) Using a graphing calculator or standard graphing software,...
-
During the course of your examination of the financial statements of the Hales Corporation for the year ended December 31, 2011, you discover the following: a. An insurance policy covering three...
-
Is the intermingling of a companys assets with those of a major shareholder, director, or company officer illegal? Is it unethical? Or is it both illegal and unethical? Explain your answer.
-
According to the SEC, BellSouths improper accounting for foreign payments in contravention of the Foreign Corrupt Practices Act (FCPA) included all of the following, except: (a) The use of fabricated...
-
According to GAAP, a company is allowed to recognize a profit in its financial statements in respect of the increase in the price of its own shares. True/False
-
According to the SECs AAER 1555, Edison disclosed the existence and amount of its District-Paid Expenses, but did not properly offset them from revenues. True/False
-
BellSouth is presented in the text as an example of the improper disclosure of non-GAAP information. True/False
-
Takada Company makes racing bicycles. Its Lightning model is considered the top of the line in the industry. Three months ago, to improve quality and reduce production time, Takada Company purchased...
-
Data on weekday exercise time for 20 females, consistent with summary quantities given in the paper An Ecological Momentary Assessment of the Physical Activity and Sedentary Behaviour Patterns of...
-
In the presence of a special type of catalyst, hydrogen gas will add across a triple bond to produce a double bond: The process is exothermic. Do you expect a high temperature to favor products or...
-
Consider the following reaction. Predict whether an increase in temperature will favor reactants or products. Justify your prediction. +
-
When an amine is protonated, the resulting ammonium ion is not electrophilic: However, when an imine is protonated, the resulting iminium ion is highly electrophilic: Explain this difference in...
-
You intend to submit a loan application to Bank of America. The loan bears a nominal annual interest rate of 18.13 percent, subject to daily compounding based on a 365-day year. Could you please...
-
SELF EVALUATION. After writing a PERSUASIVE SPEECH ON THE TOPIC: HOW TO BUILD A HAPPY MARRIAGE 1. Are you satisfied with your speech overall? Why or why not. 2. Give at least two examples of things...
-
Problem 8 a) Using the Boussinesq approach, determine the vertical stress increase beneath a point load of 200 kN at: i) depths of 1 and 3 meters directly below the point of loading ii) at the same...

Study smarter with the SolutionInn App


