Calculate S for the reduction of aluminum oxide by hydrogen gas using the following standard entropy values.
Question:
Calculate ΔS° for the reduction of aluminum oxide by hydrogen gas
![]()
using the following standard entropy values.
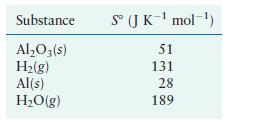
Transcribed Image Text:
AlO3(s) + 3H(g) 2Al(s) + 3HO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
AS Sproducts Sreactants 25 Als 3H...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Zirconium is one of the few metals that retains its structural integrity upon exposure to radiation. For this reason, the fuel rods in most nuclear reactors are made of zirconium. Answer the...
-
Many important biological reactions involve electron transfer. Because the pH of bodily fluids is close to 7, the biological standard potential of an electrode, E*, is measured at pH = 7....
-
Entropy can be calculated by a relationship proposed by Ludwig Boltzmann: S = kB ln V where kB = 1.38 Ã 1023 J/K and V is the number of ways a particular state can be obtained. (This equation...
-
Suppose a city finds that its express highways into the city are congested and it is considering two remedies: (1) imposing a congestion charge on all users of its expressways during the peak periods...
-
In the blizzard of 88, a rancher was forced to drop hay bales from an airplane to feed her cattle. The plane flew horizontally at 160 km/hr and dropped the bales from a height of 80m above the flat...
-
Calculate the total force on the bottom of the closed tank shown in Fig. 4.23 if the air pressure is 52 kPa(gage). Air 0,50 m Oil (sg -0.85) 0.75 m Water 18 m 1.2 m
-
In February 2007, The Elliot Group, Inc., an Illinois real estate developer, made a deal with the Village of Arlington Heights to develop property in that village. Arlington Market, LLC, was...
-
At the end of 2014, Carpenter Co. has accounts receivable of $700,000 and an allowance for doubtful accounts of $54,000. On January 24, 2015, the company learns that its receivable from Megan Gray is...
-
A broadcasting antenna emits a power of 40 W. The gain of the antenna is 300L. The receiving antenna is at a distance of 50 km from the transmitting antenna, and its effective surface area is 10-2 m....
-
Consider the reaction carried out at 25C and 1 atm. Calculate H S, and G using the following data: 2SO2(g) + O2(g) 2SO3(g)
-
Predict the sign of S for each of the following reactions. a. the thermal decomposition of solid calcium carbonate: b. the oxidation of SO 2 in air: CaCO3(s) CaO(s) + CO,(g)
-
Notes Receivable is a current liability on the balance sheet. Accept or reject. Why?
-
Suppose that the BMW Plant in Spartanburg, SC decides to replace a major assembly line on the plant floor. The current assembly line has a book value of $400,000, and the firm can sell it to another...
-
A professional role is a behavior associated with a designated function. Clients and society in general hold a variety of expectations regarding the behavior of social workers and the functions they...
-
commencement speech Now think about that day ahead when you will be receiving your diploma. If you were the graduation speaker at that event, what is the message that you would deliver to your...
-
1) What is the loss to Visa if Zelle, a P-2-P service provider, continues expanding at the rate it has been, cutting into Visa's market share? 2) Explain how the banking system works for handling...
-
You are tasked with determining how to classify leases within your organization. There are multiple leases to be considered, and you are examining the details of each using machine learning to...
-
Van Goe produces paints. On January 1, it had no work-in-process inventory. It starts production of 300,000 gallons of paint in January and completes 240,000 gallons. The costs of the resources used...
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
The pre-equilibrium and the steady-state approximations are two different approaches to deriving a rate law from a proposed mechanism. For the following mechanism, determine the rate law (a) By the...
-
(a) What is the overall reaction for the following mechanism? (b) Write the rate law based on this mechanism. (c) How will the reaction rate depend on the pH of the solution? (d) How would the rate...
-
Write the formulas for the oxoanions of the following elements in which the element is found with its highest oxidation number (see Fig. 9A.7). In each case, the charge of the oxoanion is given in...
-
1. LPs contribute 90% of the equity and fund 90% of any subsequent investments. 2. GPs contribute 10% of the equity and fund 10% of any subsequent investments. 3. All partners earn an 11% preferred...
-
Acme, Inc. instructed its bank, AJM Bank, to pay $500,000 to TreeTop, Inc. TreeTop, Inc. was also a customer of AJM Bank. AJM Bank executed the payment order by crediting Treetop's account with...
-
Rewrite this, but keep the quotes the same: It's noted that, "75% of accounting students believe that the AICPA Student Affiliate membership is helpful for their professional goals," indicating the...

Study smarter with the SolutionInn App


