Suppose it could be arranged for there to be twice as many electrons with up spins than
Question:
Suppose it could be arranged for there to be twice as many electrons with up spins than down spins in a sample in a magnetic field. Without doing any calculations, predict the sign of the temperature of such a sample. (See Exercise 4.54.)
Exercise 4.54
The populations p of the up and down spin states of electrons in a magnetic field B are given by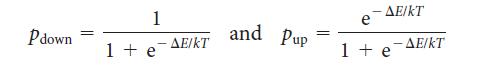
where DE = 2μBB is the energy difference between the two spin states. (See Exercise 4.53.) Plot these two populations as a function of temperature for B = 1 T.
Exercise 4.53
The molar entropy of electron spins in a magnetic field B is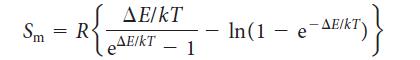
where ΔE = 2μBB is the separation in energy of the two spin states in a magnetic field, and μB is the Bohr magneton, μB = 9.274 * 10–24 J · T–1. Plot this function against temperature for the following values of B: 0.1 T, 1 T, 10 T, and 100 T.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





