It is helpful in understanding graphs of thermodynamic functions to interpret them in terms of molecular behavior.
Question:
It is helpful in understanding graphs of thermodynamic functions to interpret them in terms of molecular behavior. Consider the plot of the temperature dependence of the standard molar Gibbs free energy of the three phases of a substance in Fig. 4J.3.
(a) Explain in terms of molecular behavior why the Gibbs free energy of each phase decreases with temperature.
(b) Explain in terms of molecular behavior why the Gibbs free energy of the vapor phase decreases more rapidly with temperature than that of the solid or liquid phase.
Fig. 4J.3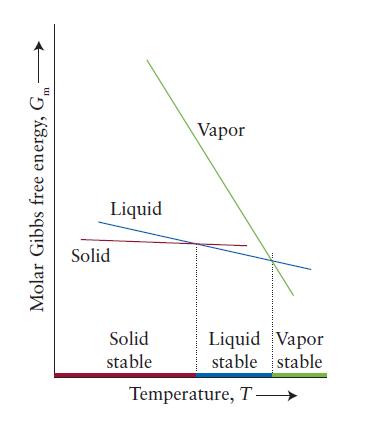
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





