The following flasks show the dissociation of a diatomic molecule, X 2 , over time. (a) Which
Question:
The following flasks show the dissociation of a diatomic molecule, X2, over time.
(a) Which flask represents the point in time at which the reaction has reached equilibrium?
(b) What percentage of the X2 molecules has decomposed at equilibrium?
(c) Assuming that the initial pressure of X2 was 0.10 bar, calculate the value of K for the decomposition.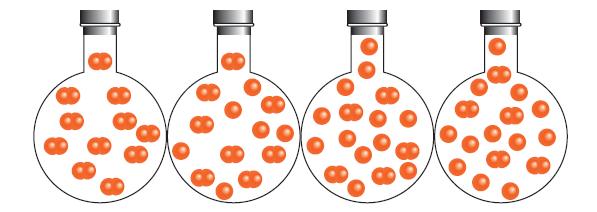
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





