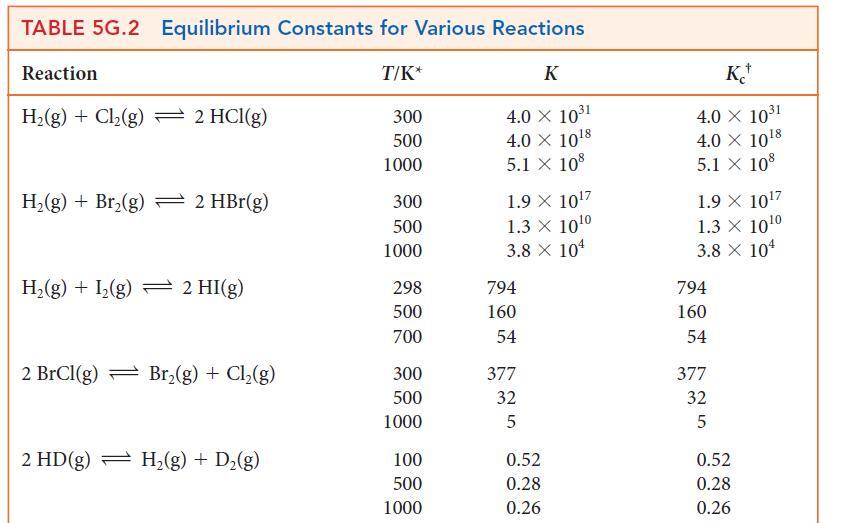
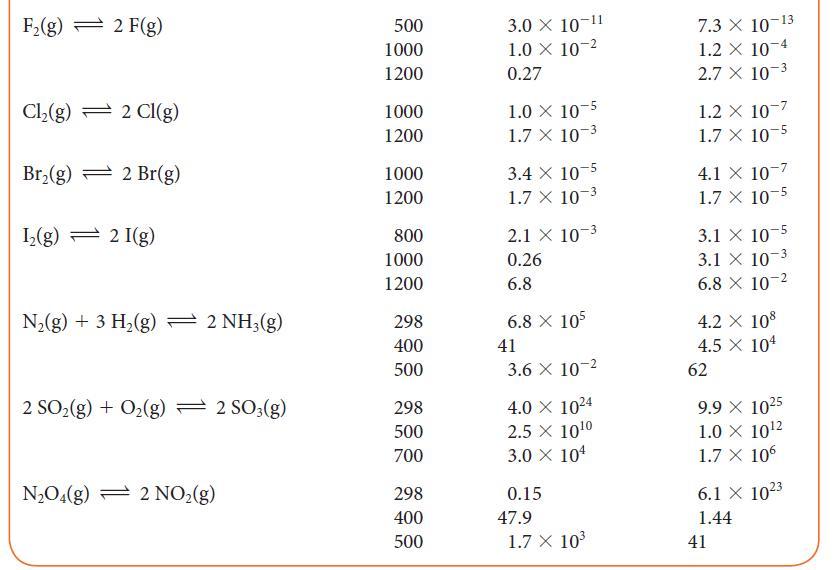
Using data from Table 5G.2 and standard graphing software, determine the standard enthalpy and entropy of the
Question:
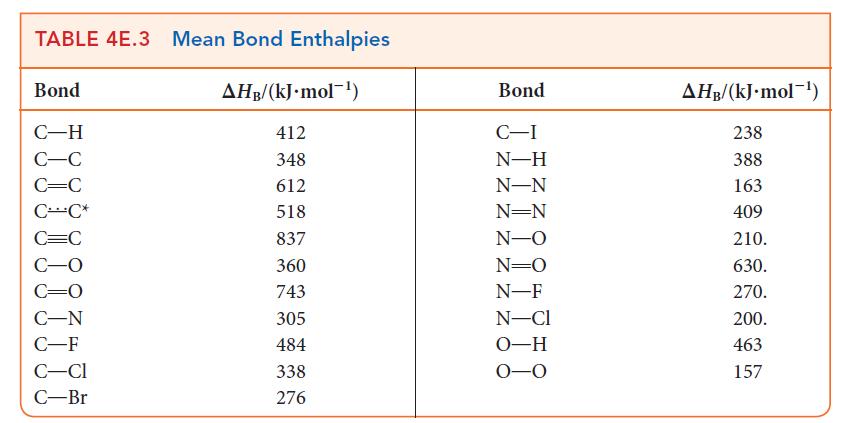
Using data from Table 5G.2 and standard graphing software, determine the standard enthalpy and entropy of the reaction N2O4(g) → 2 NO2(g) and estimate the N—N bond enthalpy in N2O4. How does this value compare with the mean N—N bond enthalpy in Table 4E.3?
Transcribed Image Text:
TABLE 4E.3 Mean Bond Enthalpies AHB/(kJ. mol) Bond C-H C-C C=C CC* C-F C-CI C-Br 412 348 612 518 837 360 743 305 484 338 276 Bond C-I N-H N-N N=N N-O N=O N-F N-Cl O-H 0-0 AHB/(kJ.mol-) 238 388 163 409 210. 630. 270. 200. 463 157
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The graph is generated for In K vs 1T according to this ...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Which of the following does not define a function? Give its domain and range. A. {(0, 1), (2, 3), (4,8)} B. y = 2x - 6 C. y = x + 2 D. X 0 3 0 6 y 1 2 2 3
-
Listed below is the number of car thefts in a large city over the last week. Calculate the coefficient of skewness using both methods. 13 7 8 3 8 3 12 3
-
The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per...
-
In Exercises 912, use the given conditions to write an equation for each line in point-slope form and general form Passing through (4, -7) and perpendicular to the line whose equation is x - 2y - 3 =...
-
An insulated glass tube and condenser are mounted on a reboiler containing benzene and toluene. The condenser returns liquid reflux so that it runs down the wall of the tube. At one point in the tube...
-
The management of Brinkley Corporation is interested in using simulation to estimate the profit per unit for a new product. Probability distributions for the purchase cost, the labor cost, and the...
-
What conditions for the binomial distribution, if any, fail to hold in the following situations? (a) For each of a company's eight production facilities, record whether or not there was an accident...
-
The following balance sheet for the Hubbard Corporation was prepared by the company: Additional information: 1. The buildings, land, and machinery are all stated at cost except for a parcel of land...
-
The figure shows the chain drive of a bicycle. How far will the bicycle move if the pedals are rotated through 180? Assume the radius of the bicycle wheel is 13.2 inches. The bicycle will travel...
-
A survey of 100 firms found the following evidence regarding profitability and market share: Is there evidence that market share and profitability are associated? Profitability Market share <15%...
-
Consider the equilibrium A (g) 2 B (g) + 3 C (g) at 25C. When A is loaded into a cylinder at 10.0 atm and the system is allowed to come to equilibrium, the final pressure is found to be 20.04 atm....
-
The three compounds methylpropene, cis-2-butene, and trans-2-butene are isomers with the formula C 4 H 8 , with G f 5 +58.07, +65.86, and +62.97 kJ mol 1 , respectively. In the presence of a...
-
An article in the Wall Street Journal about attempts by Congress to rewrite the tax code to make it more efficient noted that there were many provisions in the code intended to reduce the taxes paid...
-
The local dry cleaner is concerned about the risks from its use of chemicals, which evaporate into the air and occasionally are spilled onto the ground. The cleaner is concerned about liability for...
-
What continuous uniform series of cash flows over 7 years can be paid out of a fund having $20,000 at the present (or time 0) if interest is 10 percent compounded continuously?
-
If a potential victim can take precautions to avoid an accident (such as looking both ways before crossing a street), which liability system yields more efficient levels of precaution, strict...
-
Describe how a tax scheme could be used to regulate automobile pollution in an urban area.
-
In the early 1990 s, the chief economist of the World Bank came under a great deal of criticism for suggesting that developing countries take advantage of opportunities to import hazardous and...
-
Nathaniel purchases a house by paying $25,000 in cash and securing a home Communication Skills mortgage for $75,000. He also incurs $3,000 in legal fees, title search, and closing costs. He agrees to...
-
Write the expression in radical notation. Then evaluate the expression when the result is an integer. 23 -1/2
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic: (a) (b) (c) (d) (e) Discuss. OMe . CI H,
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
Assume that the CAPM holds. Consider a stock market that consists of only two risky securities, Stock 1 and Stock 2, with the following expected returns () and standard deviations of returns (): 1 =...
-
Explain the steps involved in the financial planning process for an individual to secure all important goals of life ?
-
Here are summary numbers from a firm's financial statements (in millions of dollars): < 2018 2019 2020 < 2021 Operating Income < 935.00 1,000.45 1,070.50 1,145.40 Net operating Assets < 6,072.25...

Study smarter with the SolutionInn App


