At 100C pure gaseous A reacts away with stoichiometry 2A R + S in a constant
Question:
At 100°C pure gaseous A reacts away with stoichiometry 2A → R + S in a constant volume batch reactor as follows: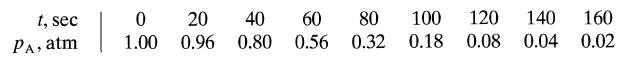
What size of plug flow reactor operating at 100°C and 1 atm can treat 100 moles A/hr in a feed consisting of 20% inerts to obtain 95% conversion of A?
Transcribed Image Text:
t, sec PA, atm 0 20 1.00 0.96 40 0.80 60 80 0.56 0.32 100 120 0.18 0.08 140 160 0.04 0.02
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To size a plug flow reactor PFR based on the desired conversion of a reactant you need to know the reaction kinetics The given data appears to relate the partial pressure of reactant A PA to the time ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Four Resistances P,Q,R,X formed a wheat stone bridge. The bridge is balanced when R=1002. If P and Q are inter changed the bridge balance for 1212 The value of x is 4. 1) 1002 2) 2002 3) 3002 4) 1102...
-
Repeat the previous problem for a mixed flow reactor. The data in Table P5.28 have been obtained on the decomposition of gaseous reactant A in a constant volume batch reactor at 100C. The...
-
The data in Table P5.28 have been obtained on the decomposition of gaseous reactant A in a constant volume batch reactor at 100C. The stoichiometry of the reaction is 2A R + S. What size plug flow...
-
Elevator Controller The block diagram for an elevator controller for a two-floorelevator follows. The inputs FB1 and FB2 are 1 when someone in the elevatorpresses the first and secondfloor buttons,...
-
Distinguish between adverse selection and moral hazard as they relate to the insurance industry.
-
The Excel worksheet form that follows is to be used to recreate the main example in the text related to the Colonial Pewter Company. Download the workbook containing this from Connect. On the website...
-
Data set: Daily calorie intakes (in kilojoules) of 28 people Construct a cumulative frequency distribution and an ogive for the data set using six classes. Then describe the location of the greatest...
-
A local pizza shop has hired a consultant to help it compete with national chains in the area. Because these national chains handle most business, the local shop operates as a price taker. Using...
-
The costs per equivalent unit of direct materials and conversion in the Rolling Department of Jabari Steel Company are $1.30 and $1.85, respectively. The equivalent units to be assigned costs are as...
-
We wish to treat 10 liters/min of liquid feed containing 1 mol A/liter to 99% conversion. The stoichiometry and kinetics of the reaction are given by Suggest a good arrangement for doing this using...
-
Originally we had planned to lower the activity of a gas stream containing radioactive Xe-138 (half-life = 14 min) by having it pass through two holdup tanks in series, both well mixed and of such...
-
Cas Experiment, Undetermined Coefficients Since variation of parameters is generally complicated; it seems worthwhile to try to extend the other method. Find out experimentally for what ODEs this is...
-
What is coercive power? Why is it not considered a form of leadership? What is the difference between socialized and personalized charismatic leaders? Explain the concept of competitive advantage and...
-
Describe the shape and factors that affect the demand curve and the supply curve in a market. Discuss the concept of market equilibrium and how changes in demand and supply affect the equilibrium...
-
Impact of current global financial crisis on CIMB Group. -use the annual report as a reference -look at articles to elaborate
-
Explain the relationship between compound interest and exponential growth.? provide Example
-
Can the conceptual framework of bankruptcy be reconceptualized through interdisciplinary lenses, incorporating insights from behavioral economics, game theory, and institutional economics, to...
-
Corporate warfare can get nasty. One firm may decide to acquire another. How does it do this? What can the target corporation do to prevent its being taken over? Should stockholders of either...
-
In Exercises, find the equation of the tangent line at the given point on each curve. 2y 2 - x = 4; (16, 2)
-
Read through all the problems at the end of this chapter. Make up and solve an original problem based on the material in this chapter. (a) Use real data and reactions for further instructions. (b)...
-
List two similarities and two differences between the Safety Analysis of the Incident Algorithm and the BowTie Diagram. a. Example 6-1: Gas-Phase Reaction in a Microreactor Wolfram and Python 1. Use...
-
Download the Interactive Computer Games (ICG) from the CRE Web site (http://www.umich.edu/~elements/6e/icm/tictac.html). Play the game and then record your performance number, which indicates your...
-
g(x) we derived rudimentary formulae for the centered differences of the first and second derivatives. These were based on Taylor approximations for m1. Regardless, you can improve on these estimates...
-
10000 counts were obtained from a 5min count of a radioactive source. The background was 4000 counts in 10 minutes. Q4A. What is the net count rate of the source with absolute uncertainty?
-
We are designing a process unit for the isomerization of pentanol: It is notably packed with catalyst pellets. The radius of the catalyst pellets is 2.14 mm. Besides, their reaction coefficient is...

Study smarter with the SolutionInn App


