Aluminum crystallizes with a face-centered cubic unit cell. The radius of an aluminum atom is 143 pm.
Question:
Aluminum crystallizes with a face-centered cubic unit cell. The radius of an aluminum atom is 143 pm.
Calculate the density of solid crystalline aluminum in g/cm3.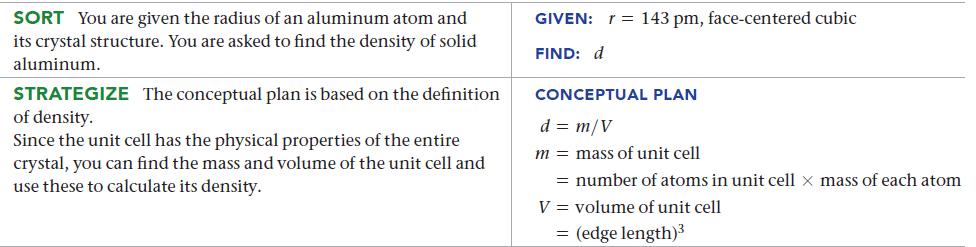
Transcribed Image Text:
SORT You are given the radius of an aluminum atom and its crystal structure. You are asked to find the density of solid aluminum. STRATEGIZE The conceptual plan is based on the definition of density. Since the unit cell has the physical properties of the entire crystal, you can find the mass and volume of the unit cell and use these to calculate its density. GIVEN: FIND: d r = 143 pm, face-centered cubic CONCEPTUAL PLAN d = m/V m = mass of unit cell = number of atoms in unit cell x mass of each atom V = volume of unit cell = (edge length)³
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
1 mol g mAl atom 2698 X mol 6022 x 1023 atoms 4480 ...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Restart exam Which statement is not correct under the Circular No. 230 rule on conflicts of interests? O The practitioner may represent a client despite having a conflict of interest if certain...
-
A crystalline form of molybdenum has a face centered cubic structure. If the radius of a molybdenum atom is 139 pm, calculate its density. (hint): answer should be 10.3 g/cm 3
-
Boron phosphide, BP, is a semiconductor and a hard, abrasion-resistant material. It is made by reacting boron tribromide and phosphorus tribromide in a hydrogen atmosphere at high temperature (>750...
-
A non reactive/conservative contaminant is dumped on the ground level and it leaches to the groundwater vertically and takes half day for reaching the groundwater by travelling through unsaturated...
-
Vance was served liquor while he was an intoxicated patron of the Clear Air Force Station Non-Commissioned Officers Club. He later injured himself as a result of his intoxication. An Alaska State...
-
A generic diagram of a slope in a jointed rock mass that is threatened by a planar block slide is shown, in the sketch. Although not shown, bench height is 55 ft (16.76 m). Given that MohrCoulomb...
-
If your instructor assigns a marketing plan for your class, we hope you will be excitedfor two reasons. First, you will get insights into trying to actually do marketing that often go beyond what you...
-
RETAINED EARNINGS APPROPRIATION On October 2, 20-1, the board of directors of Foxworth Company appropriated $80,000 of retained earnings for the purpose of buying a new sailboat (used for...
-
Unusual shapes! At this point, we've gotten pretty comfortable with calculating the perimeter and area of squares and rectangles. In this assignment, we'll make a program that calculates these values...
-
Which solid would you expect to have the highest melting point? (a) MgO(s) (b) I 2 (s) (c) Kr(s)
-
A body-centered cubic unit cell has a volume of 4.32 * 10 - 23 cm 3 . Find the radius of the atom in pm.
-
Solve each of the following equations. x + 0.07x = 64.20
-
1. Ronnie Gilley Properties, LLC (Gilley), wrote a check to Cile way Properties, LLC (Cile), for $100,000. when Cile deposited the check in its bank, it was misencoded in the amount of $1,000. As a...
-
After a series of all-day meetings with software vendors, the CEO, COO and CFO of a hospital evaluated their options and decided on a course of action. As they began implementing the new electronic...
-
A newly married couple is looking for a new 3-bedroom house in London. They surveyed 12 houses they liked and recorded the price and the distance from the nearest station of those houses. The data is...
-
As a rule of thumb, what assets should be used FIRST during retirement so as to minimize the tax liability for spouses/common-law partners? a) non-registered assets of the higher income spouse or...
-
Question The digital microwave radio system shown in Figure 2 operates in... The digital microwave radio system shown in Figure 2 operates in the 18GHz radio frequency band and provides 2x2 Mbps...
-
The following December 31, 2013, fiscal year-end account balance information is available for the Stonebridge Corporation: Cash and cash equivalents........ $ 5,000 Accounts receivable (net)...
-
What steps must a business take to implement a program of social responsibility?
-
The chlorination of vinyl chloride, C 2 H 3 Cl + Cl 2 C 2 H 3 Cl 3 , is believed to proceed by the following mechanism: Derive the rate law expression for the chlorination of vinyl chloride based on...
-
In the unimolecular isomerization of cyclobutane to butylene, the following values for kuni as a function of pressure were measured: Assuming that the Lindemann mechanism accurately describes this...
-
In the discussion of the Lindemann mechanism, it was assumed that the rate of activation by collision with another reactant molecule, A, was the same as collision with a nonreactant molecule, M, such...
-
Suppose back in 2000, the average ticket price of the Houston Symphony was $66.9 years later, the average ticket price increased to $81. What was the growth rate of the average ticket price over the...
-
Suppose a bank pays a quoted annual (simple) interest rate of 9%. However, it pays interest (compounds) daily using a 365-day year. What is the effective annual rate of return (EAR)?(Round the answer...
-
Suppose you read in The Wall Street Journal that a $1,000 par value, 8 year, 6%, semi-annual bond is trading at $984. If your required rate of return is 6%, what would be the bond's current yield?...

Study smarter with the SolutionInn App


