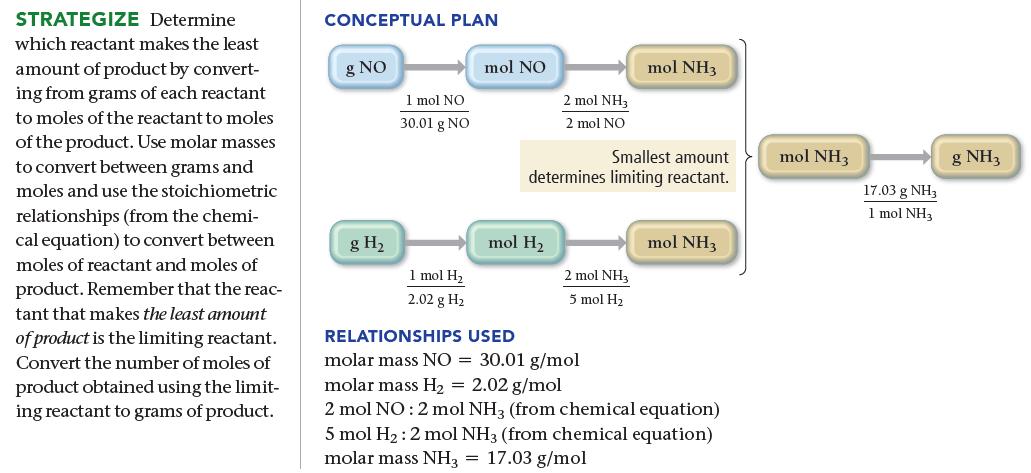
Ammonia, NH 3 , can be synthesized by the reaction: Starting with 86.3 g NO and 25.6
Question:
Ammonia, NH3, can be synthesized by the reaction:![]()
Starting with 86.3 g NO and 25.6 g H2, find the theoretical yield of ammonia in grams.
Transcribed Image Text:
2 NO(g) + 5 H₂(g) 2 NH3(g) + 2 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
863 g NO X Limiting reactant 1 mol NO 2 mol NH3 3001 g NO 2 melNO 256 ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Ammonia, NH3, can be synthesized by the reaction: 2 NO(g) + 5 H2(g) -------> 2 NH3(g) + 2 H2O(g) Starting with 96.4 g NO and 35.8 g H2, find the theoretical yield of ammonia in grams.
-
Nitrogen dioxide NO 2 can be synthesized by the reaction: N 2 (g) + 2 O 2 (g) 2 NO 2 (g) Starting with 46.3 g N 2 and 92.0 g O 2 , find the theoretical yield of NO 2 in grams.
-
The following compounds can be synthesized by aldol condensations, followed by further reactions. (In each case, work backward from the target molecule to an aldol product, and show what compounds...
-
A company has the following data: net sales, $405,000; cost of goods sold, $220,000; selling expenses, $90,000; general and administrative expenses, $60,000; interest expense, $4,000; and interest...
-
The financial statements of Tootsie Roll Industries are presented in Appendix A. Instructions Answer the following questions. (a) What was the amount of net cash provided by operating activities for...
-
Under steady-state operation the surface temperature of a small 20-W incandescent light bulb is 125C when the temperature of the room air and walls is 25C. Approximating the bulb as a sphere 40 mm in...
-
One critical-thinking skill is a heightened awareness of the danger of reaching a conclusion prior to acquiring missing information that were it known would have a reasonable probability of altering...
-
A six-column table for JKL Company follows. The first two columns contain the unadjusted trial balance for the company as of July 31, 2013. The last two columns contain the adjusted trial balance as...
-
It is now June 30, 2023. The journal entry for the investment in the put option had already been recorded on January 1, 2023. SmartiePants Corp prepared its financial statements on March 31, 2023 as...
-
Propose a research strategy to investigate Natalie Churyks suggestions for mastering the computerized CPA exam. Then implement the strategy and communicate the results (citing the relevant source).
-
Nitrogen and hydrogen gas react to form ammonia according to the reaction: A flask contains a mixture of reactants represented by the image shown at the left. Which of the following images best...
-
Sodium and chlorine react to form sodium chloride: What is the theoretical yield of sodium chloride for the reaction of 55.0 g Na with 67.2 g Cl 2 ? a) 1.40 * 10 2 g NaCl b) 111 g NaCl c) 55.4 g NaCl...
-
CH 4 belongs to the T d point group with the following symmetry elements: E, 4C 3 , 4C 2 3 , 3C 2 , 3S 4 , 3S 3 4 , and 6Ï. Make a drawing similar to Figure 27.1 showing these elements. C2, S....
-
Discuss McLuhan's Laws of Media and assess whether it is a viable scientific theory of socio-technological evolution ?
-
Consider a three-security portfolio below: Security 1 2 3 Maturity (in years) 1 1 1 Par value Price Yield to maturity $100 $98 2.06% $100 $95 5.0% $100 $97 3.1% These securities pay $0 coupons at the...
-
What effect has legal changes that allow more consolidation of media under major corporations had? Do we need new laws limiting media ownership in the U.S.? Why? Use specific examples in your...
-
Pick one piece of the American Dream Malls' history and discuss which project knowledge domains are revealing ad explains why in an essay. Or pick one domain that was consistently done well or poorly?
-
2 small breifing of media law case studies of malaysia ?
-
After the amount due on a sale of $40,000, terms 2/10, n/eom, is received from a customer within the discount period, the seller consents to the return of the entire shipment. The cost of the...
-
Citing a scientific article, explain in your own words, how DNA fingerprinting has been used in forensic science to solve crimes and why it may not always be accurate or effective.
-
You have collected a tissue specimen that you would like to preserve by freeze drying. To ensure the integrity of the specimen, the temperature should not exceed 5.00C. The vapor pressure of ice at...
-
The phase diagram of NH 3 can be characterized by the following information. The normal melting and boiling temperatures are 195.2 and 239.82 K, respectively; the triple point pressure and...
-
Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical method or a least squares fitting routine. T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230....
-
Identify the entities and the relationships the diagram given below Translate the Diagram Into Relational Model Fname Minit Lname San Bdate Sex No Street Apt no City State Zip Name PERSON Address...
-
A 2 kg block is attached to a spring with a force constant of 400 N/m. The block is initially at rest and is compressed by 0.5 meters from its equilibrium position. When released, the block undergoes...
-
Map this ER diagram to a Relational Model and normalize it if needed. employee EMP_ID INT EMP_Frame VARCHAR(40) EMP_Lname VARCHAR(40) EMP_Sex VARCHAR(1) EMP_Birthdate DATE EMP_Salary INT Indexes...

Study smarter with the SolutionInn App


