Below is a representation of 50 atoms of a fictitious element called pearsonium (Ps). The red spheres
Question:
Below is a representation of 50 atoms of a fictitious element called pearsonium (Ps). The red spheres represent Ps-296, the blue spheres Ps-297, and the green spheres Ps-298.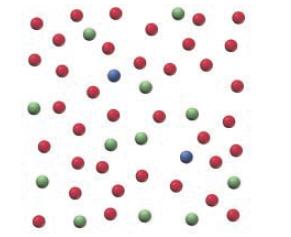
a. Assuming that the sample is statistically representative of a naturally occurring sample, calculate the percent natural abundance of each Ps isotope.
b. Draw the mass spectrum for a naturally occurring sample of Ps.
c. The mass of each Ps isotope is measured relative to C-12 and tabulated. Use the mass of C-12 to convert each of the masses to amu and calculate the atomic mass of Ps.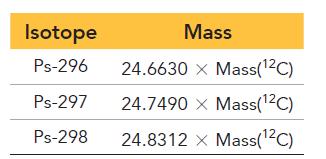
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





