Consider the densities and atomic radii of the noble gases at 25 C: a. Estimate the densities
Question:
Consider the densities and atomic radii of the noble gases at 25 °C: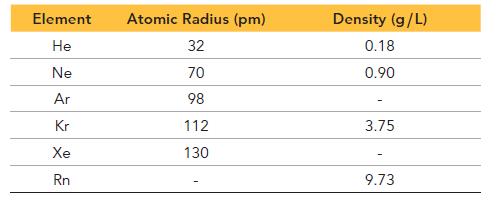
a. Estimate the densities of argon and xenon by interpolation from the data.
b. Estimate the density of the element with atomic number 118 by extrapolation from the data.
c. Use the molar mass of neon to estimate the mass of a neon atom. Then use the atomic radius of neon to calculate the average density of a neon atom. How does this density compare to the density of neon gas? What does this comparison suggest about the nature of neon gas?
d. Use the densities and molar masses of krypton and neon to calculate the number of atoms of each element found in a volume of 1.0 L. Use these values to estimate the number of atoms present in 1.0 L of Ar. Now use the molar mass of argon to estimate the density of Ar. How does this estimate compare to that in part a?
Step by Step Answer:






