Consider the reaction: The following data show the equilibrium constant for this reaction measured at several different
Question:
Consider the reaction:
![]()
The following data show the equilibrium constant for this reaction measured at several different temperatures. Use the data to find ΔH°rxn and ΔS°rxn for the reaction.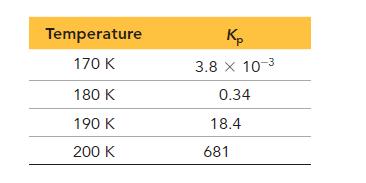
Transcribed Image Text:
2 NO(g) + O₂(g) = 2 NO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The image you sent shows the following reaction 2 NOg O2g 2 NO2g This is the reaction of nitrogen monoxide and oxygen to form nitrogen dioxide It is a...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction: The following data show the equilibrium constant for this reaction measured at several different temperatures. Use the data to find H rxn and S rxn for the reaction. H(g) +...
-
Using the FCFS rule for scheduling, the sequence is 1-2-3 For the schedule developed using the FCFS nuis, the total length of time taken to complete the three sults (including delivery) 22 hours...
-
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction....
-
An undamped mass spring system is released from rest from an initial displacement of x = 0.24 m and starts to oscillate. You see that the mass reaches its largest positive displacement for the first...
-
On June 30, New Haven Companys work in Process inventory account showed a beginning balance of $29,400. The Materials Inventory account showed a beginning balance of $240,000. Production activity for...
-
Betsy started Betsys Flag Co. on September 1. During September, Betsy consumed the following resources: Rent ............$ 625 Utilities ........... 250 Supplies ..........3,000 Repairs...
-
Repeat the calculations of Example 6.3, but for \(80 \mathrm{~mol} \%\) of the liquid distilled. Data From Example 6.3:- Suppose the liquid of Example 6.1 [50 mol% n-heptane (A), 50 mol% n-octane...
-
The following information is available for Sedona, Inc., as of May 31, 2014: a. Cash on the books as of May 31 amounted to $42,754.16. Cash on the bank statement for the same date was $52,351.46. b....
-
The adjusted trial balance for Chiara Company as of December 31 follows. Cash Accounts receivable Interest receivable Debit $ 30,000 Credit 52,000 18,000 nt ok Notes receivable (due in 90 days)...
-
The change in enthalpy (H rxn ) for a reaction is -25.8 kJ/mol. The equilibrium constant for the reaction is 1.4 * 10 3 at 298 K. What is the equilibrium constant for the reaction at 655 K?
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P CH3OH = 1.0 atm; P CO = P H2 = 0.010 atm CO(g) +...
-
The cost formula for the maintenance department of Rainbow, Ltd., is $19,400 per month plus $7,70 per machine hour used by the production department. Required: a. Calculate the maintenance cost that...
-
Kofas firms are always looking for methods to improve efficiency and effectiveness to compete globally. Some turn to mergers and acquisitions to survive. Thus, NewGold and Powell want to unite to...
-
To get a loan, Black Mountain Brewery grants a security interest in its accounts receivable from customers. In later litigation, the case turns on whether the accounts receivable are "accounts," as...
-
Suppose the eighth unit of a product cost is $850. What is the expected cost of the 128th unit if the product follows a 75% learning rate?
-
Use your knowledge of cost functions to calculate the missed cost data in the accompanying table. Round your answers to two digits after the decimal. Quantity Marginal cost Fixed cost Variable cost...
-
An aqueous solution containing 15% NaOH and 0.5% NaCl is concentrated at a rate of 100 kg/min in a evaporator. The concentrated solution is then mixed with a 2000 kg of aqueous solution of NaOH...
-
Why is the relationship between the buyer and seller increasingly important to successful B2B marketing practices?
-
What does non-recourse financing mean?
-
Consider a Cavendish apparatus that employs a torsion fiber with k = 1.0 10 -8 N m. Suppose the smallest twist angle that can be measured is = 2.0. If the distance from one of the masses m 1 to...
-
Figure P11.62 shows the amplitude as a function of frequency for a driven, damped oscillator. Estimate the resonant frequency. Figure P11.62 ? 500 1000 1500 Frequency (Hz) Amplitude (mm)
-
Figure P11.61 shows the displacement as a function of time for an under-damped harmonic oscillator. Estimate the fraction of the mechanical energy that is ?lost? to friction during one cycle. Figure...
-
Rick & Morty conduct business as partners operating a toy store. Their partnership agreement specifies that all profits and losses are to be distributed equally after partners salaries. During the...
-
Phil, a resident taxpayer, purchased a residential apartment as an investment from which he derived rental income. During the 2023 income year, Phil incurred the following outgoings in connection...
-
Accounting 1C Long Term Project- Master Budget You need to prepare a Master Budget for the The company has an exclusive right to sell PowerPulses and sales have been brisk. The Master Budget will be...

Study smarter with the SolutionInn App


