Consider the titration curves (labeled a and b) for two weak acids, both titrated with 0.100 M
Question:
Consider the titration curves (labeled a and b) for two weak acids, both titrated with 0.100 M NaOH.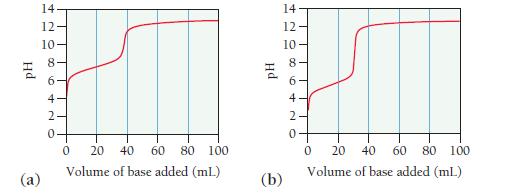
i. Which acid solution is more concentrated?
ii. Which acid has the larger Ka?
Transcribed Image Text:
Hd @ 14 12- 10 08 6 4- 420 20 40 60 80 100 Volume of base added (ml.) 0 Hd (b) 14 12 10 0 20 40 60 80 100 Volume of base added (mL) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
i...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a company issues shares of stocks it is a. Adding to the company's debts. b. Investors in the stocks are extending loans to the company. c. Issuing shares of ownership in the company. d. It is...
-
Two 25.0-mL samples of unknown monoprotic weak acids, A and B, are titrated with 0.100 M NaOH solutions. The titration curve for each acid is shown below. Which of the two weak acid solutions is more...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
On September 1, 2025, Swifty Corporation acquired Windsor Enterprises for a cash payment of $800,000. At the time of purchase, Windsor's balance sheet showed assets of $570,000, liabilities of...
-
Sieber has prepared the following list of statements about decision making and incremental analysis. 1. The first step in managements decision-making process is, Determine and evaluate possible...
-
The following revenue and expense account balances were taken from the ledger of Graphics Services Co. after the accounts had been adjusted on February 29, 2012, the end of the current fiscal year:...
-
What would happen to the SML graph in Figure 8.8 if expected inflation increased or decreased? Figure 8.8 268 269 270 271 272 273 274 275 A Required Rate of Return TH-13.0% SML: r, RF+RPM * b D E F H...
-
Book versus Tax Depreciation Griffith Delivery Service purchased a delivery truck for $33,600. The truck has an estimated useful life of six years and no salvage value . For purposes of preparing...
-
1. The capital market is a market prepared for trading stocks, securities. The capital market acts as a liaison between investors and companies or government institutions through long-term trading...
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
Consider the titration of a 25.0-mL sample of 0.175 M CH 3 NH 2 with 0.150 M HBr. Determine each quantity. a. The initial pH b. The volume of added acid required to reach the equivalence point c. The...
-
If f is continuous on ( , ), what can you say about its graph?
-
In 1854, Mexico sold the United States roughly 30,000 square miles of land known as the Gadsden Purchase for $0.003 per square mile. In 2019, the average value of an acre at this location is $5000....
-
What are some of the arguments (by Indian policymakers and economists) in favor of demonetization? present the pros and the cons. Do you think these are short-run adjustments or a more serious...
-
2. Describe a situation in which a county health officer may want to know the count of expected cases of a condition in the county rather than a rate? (3 points)
-
Explain the factors that influence managerial ethics using your own examples.
-
Actual Carry Trade The interest rate is 1% in country A, and 6% in B, and the exchange rate is 1A = 2B (you need 2 of B to buy 1 of A). You begin a 1-year Carry Trade by borrowing 1000 units of...
-
Explain the economic benefit doctrine.
-
If a force of F = 50 Ib is applied to the pads at A and C, determine the smallest dimension d required for equilibrium if the spring has an unstretched length of 1 ft. B 1 ft 1 ft F k = 15016/fr 1ft...
-
A Michelson Interferometer is illuminated with monochromatic light. One of its mirrors is then moved 2.35 10 -5 m, and it is observed that 92 fringe-pairs, bright and dark, pass by in the process....
-
Examining photos of Newtons rings we observe that fringes at large values of m seem to be nearly equally spaced. To see that analytically, show that When m is large, the spacings between consecutive...
-
When dust gets between the glass elements of a Newtons ring setup, it can cause an unknown shift in the film thickness Îd, and a corresponding change in the fringe pattern. The path difference...
-
8. In the game shown, player 1 learns his type and sends a message, and then both players 1 and 2 simultaneously choose actions. Find a separating per- fect Bayes-Nash equilibrium. 1 W 2 a Type s...
-
1) Do you think lobbying is a beneficial organization group for some, or is it just "legalized bribery" which should be made illegal? 2) Does privatization of certain government provided services...
-
What is Foreign Direct Investment (FDI): Disney, a US-based company, is investing in India, which is considered a foreign direct investment. FDI is a critical driver of economic growth for developing...

Study smarter with the SolutionInn App


