Copper has two naturally occurring isotopes with masses 62.94 amu and 64.93 amu and has an atomic
Question:
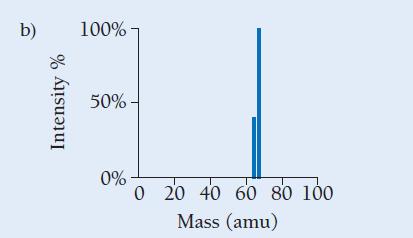
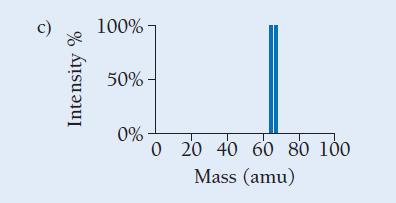
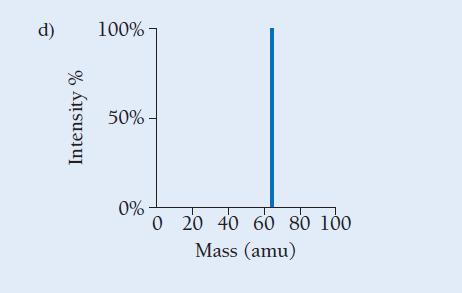
Copper has two naturally occurring isotopes with masses 62.94 amu and 64.93 amu and has an atomic mass of 63.55 amu. Which mass spectrum (of those shown at right) is most likely to correspond to a naturally occurring sample of copper?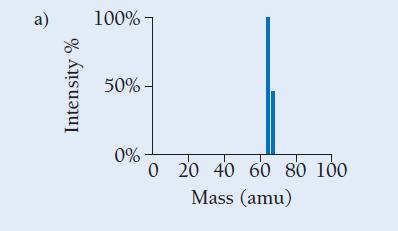
Transcribed Image Text:
a) Intensity % 100% 50% 0% 0 20 40 60 80 100 Mass (amu)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Intensi...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
278+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An element has two naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 49.9472. 2.500 103 50.9440. 0.9975 What is the atomic mass of this...
-
(A) The masses and percent isotopic abundances of the three naturally occurring isotopes of silicon are 28 Si, 27.9769265325 u, 92.223%; 29 Si, 28.976494700 u, 4.685%; 30 Si, 29.973377017 u, 3.092%....
-
Gallium (Ga) consists of two naturally occurring isotopes with masses of 68.926 and 70.925 amu. (a) How many protons and neutrons are in the nucleus of each isotope? Write the complete atomic symbol...
-
Steve and Linda Hom live in Bartlesville, Oklahoma. Two years ago, they visited Thailand. Linda, a professional chef, was impressed with the cooking methods and the spices used in the Thai food....
-
Star City is considering an investment in the community center that is expected to return the following cash flows: Year Net Cash Flow 1 . . . . . . . . . . . . $ 20,000 2 . . . . . . . . . . . ....
-
Find the de Broglie wavelength of hydrogen molecules, which corresponds to their most probable velocity at room temperature.
-
In 1951, DuPont began using the chemical perfluorooctanoic acid to manufacture Teflon. Due to the dangerous nature of the chemical, DuPont was given special instructions by its supplier to dispose of...
-
The Bennet Company purchases one of its essential raw materials from three suppliers. Rennets current policy is so distribute purchases equally among the three. The owners son, Benjamin Rennet, just...
-
15. Jenny borrows $20,000 for her car at an interest rate of 2.5% to be paid off over five years, during which time the inflation rate averages 7%
-
There is a database that contains a website's traffic data over a period of 30 days. The first table contains users' information including the user type (user, crawler, admin). The second table...
-
Explain Millikans oil drop experiment and how it led to the measurement of the electrons charge. Why is the magnitude of the charge of the electron so important?
-
A thief uses a can of sand to replace a solid gold cylinder that sits on a weight-sensitive, alarmed pedestal. The can of sand and the gold cylinder have exactly the same dimensions (length = 22 and...
-
Express in simplest radical form with a rationalized denominator. 3-1/2 2
-
Which will reduce cash outflows that support lifestyle choices? I. Being a big saver for retirement. II. Adopting a pay-yourself-first approach. III. Paying down debt aggressively. Question 10Select...
-
What is the main advantage of a line of credit over an instalment loan? Question 23Select one: a. Amount repaid can be reborrowed b. Payments are fixed and predictable c. The interest rate is fixed...
-
What are three (3) standard timeframes that apply to production and review of budgets? Why and how also Discus each timeframes in detail.
-
Utilizing resoorces, complete a risk audit of Bank of Americas project to acquire Merrill Lynch. Ensure you address all 10 critical success factors. From the evidence of your audit, would you advise...
-
Write a program to calculate the total price for car wash services. A base car wash is $10. The cost for additional services are as follows: Air freshener Rain repellent Tire shine Wax Vacuum $1 $2...
-
Terry Manufacturing Company was started on January 1, 2015, when it acquired $2,500 cash by issuing common stock. During its first year of operation, it purchased $500 of direct raw materials with...
-
Classify each of the following as direct costs or indirect costs of operating the Pediatrics ward for children at the Cleveland Clinic: a. Wi-Fi covering the entire hospital campus b. Net cost of...
-
Calculate H and S if the temperature of 1.75 moles of Hg(l) is increased from 0.00 o C to 75.0 o C at 1 bar. Over this temperature range, C P,m = (J K -1 mol -1 ) 30.093 4.944 10 -3 T/K.
-
Draw the mechanism for each of the following reactions: a. b. c. NaOMe CI NaOEt. Br
-
In the next chapter, we will learn a method for preparing alkynes (compounds containing C ¡ C triple bonds). In the following reaction, a dihalide (a compound with two halogen atoms) is treated...
-
A marketer is deciding whether the marketing campaign for his company's product will be a push strategy, a pull strategy, or a combination of the push/pull strategies. What impact does this decision...
-
The dedicated production line with the following figures Production setup cost ($/setup) Holding cost ($ for each PCS/year) Demand (PCS/year) Capacity (PCS/year) 100 0.90 7,000 34,000 a. Determine:...
-
Let p be a prime. = 0 for 0 0 and gcd (p, b) = 1. 1+cph+1 with gcd(p, c) = 1, unless p = 2 and h = 1. ap [10 pts] Show that: If p is odd and e> 0, then: (c) In (Zpe), [1 + p] has order pe-1; ->. and...

Study smarter with the SolutionInn App


