Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals,
Question:
Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 11.6 and 11.7.
a. COCl2 (carbon is the central atom)
b. BrF5
c. XeF2
d. I3–
Examples 11.6
Write a hybridization and bonding scheme for bromine trifluoride, BrF3.
Examples 11.7
Write a hybridization and bonding scheme for acetaldehyde,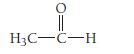
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





