Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write
Question:
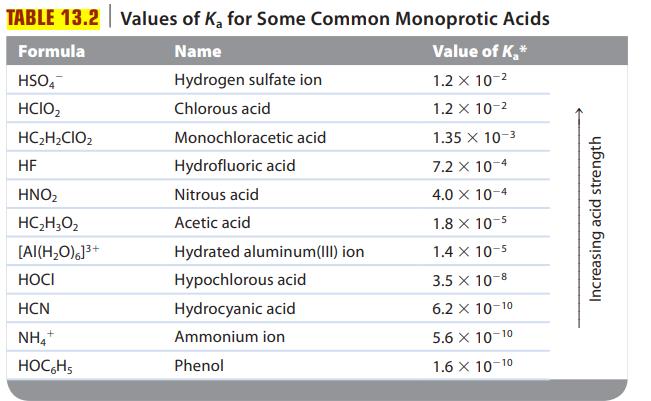
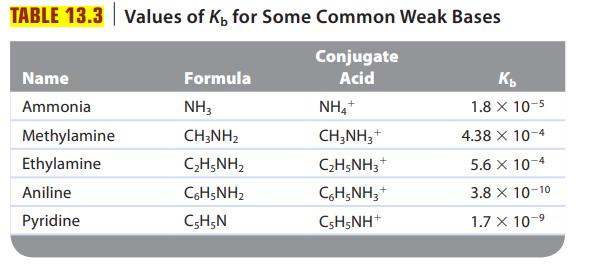
Are solutions of the following salts acidic, basic, or neutral? For those that are not neutral, write balanced equations for the reactions causing the solution to be acidic or basic. The relevant Ka and Kb values are found in Tables 13.2 and 13.3.
Transcribed Image Text:
TABLE 13.2 Values of K₂ for Some Common Monoprotic Acids Name Value of K₂* Hydrogen sulfate ion 1.2 x 10-² Chlorous acid 1.2 x 10-² 1.35 x 10-3 7.2 x 10-4 4.0 X 10-4 Formula HSO4 HCIO₂ HC₂H₂CIO₂ HF HNO₂ HC,H,Oz [AI(H₂O)]³+ HOCI HCN NH4+ HOCHS Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol 1.8 x 10-5 1.4 x 10-5 3.5 x 10-8 6.2 X 10-10 5.6 X 10-10 1.6 X 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Solutions of the following salts are acidic basic or neutral Salt Solution Equation NaHS Basic NaHS H2O HS Na H3O NH4Cl Acidic NH4Cl NH4 Cl NaCH3COO B...View the full answer

Answered By

Joemar Canciller
I teach mathematics to students because I love to share what I have in this field.
I also want to see the students to love math and be fearless in this field.
I've been tutoring these past 2 years and I would like to continue what I've been doing.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Lets take a look at the extensive career of Helen Frankenthaler (1928-2011), who found a way to incorporate all of the innovative techniques artists were experimenting with starting in 1945. Her...
-
Will 0.10 M solutions of the following salts be acidic, basic, or neutral? a. Ammonium bicarbonate b. Sodium dihydrogen phosphate c. Sodium hydrogen phosphate d. Ammonium dihydrogen phosphate e....
-
Decide whether solutions of the following salts are acidic, neutral, or basic. a. Ammonium acetate b. Anilinium acetate
-
Which of the following would be the most frequently occurring daily transaction in a retail shop? (a) Paying salary to the sales assistant (b) Sale of goods (c) Payment of rent for the shop premises...
-
James Lillards first wife had a child whom James adopted when he married that childs mother. James fathered other children with her until they divorced in the early 1970s. In 1975, James married his...
-
Suppose you receive $100 at the end of each year for the next three years. a. If the interest rate is 8%, what is the present value of these cash flows? b. What is the future value in three years of...
-
Which intangible asset is recorded only as part of the acquisition of another company? a. Copyright b. Patent c. Franchise d. Goodwill
-
A 10-year project has an initial fixed asset investment of $38,640, an initial NWC investment of $3,680, and an annual OCF of -$58,880. The fixed asset is fully depreciated over the life of the...
-
Find five parliamentary countries and examine how heads of state are selected in those countries. How powerful are the heads of state in those countries?
-
Enlightened Eats in Anchorage, Alaska, has six employees who are paid semimonthly. Calculate the net pay from the information provided below for the November 15 pay date. Assume that all wages are...
-
Consider a solution of an unknown salt having the general formula BHCl, where B is one of the weak bases in Table 13.3. A 0.10-M solution of the unknown salt has a pH of 5.82. What is the actual...
-
The K b values for ammonia and methylamine are 1.8 10 -5 and 4.4 10 -4 , respectively. Which is the stronger acid, NH 4 + or CH 3 NH 3 + ?
-
Multiply out the brackets: (a) 7(x y) (b) 3(5x 2y) (c) 4(x + 3) (d) 7(3x 1) (e) 3(x + y + z) (f) x(3x 4) (g) y + 2z 2(x + 3y z)
-
Compare both the models qualitatively.
-
Write CRC cards for accessibility SAP.
-
Write four challenges of using accessibility SAP.
-
Compare traditional accessibility model with accessibility SAP. Which one do you like more?
-
Discuss the nonfunctional requirements for accessibility SAP.
-
On January 1, 2016, DIBA Company had a balance of $450,000 in its Bonds Payable account. During 2016, DIBA issued bonds with a $200,000 face value. There was no premium or discount associated with...
-
Starr Co. had sales revenue of $540,000 in 2014. Other items recorded during the year were: Cost of goods sold ..................................................... $330,000 Salaries and wages...
-
Draw the structure of the product with molecular formula C 10 H 10 O that is obtained when the compound below is heated with aqueous acid. CN CN C10H100 Heat
-
Lactones can be prepared from diethyl malonate and epoxides. Diethyl malonate is treated with a base, followed by an epoxide, followed by heating in aqueous acid: Using this process, identify what...
-
Predict the major product of the following transformation. CO2ET C10H100 Heat
-
Choose a market that has been impacted by the Russia-Ukraine and Israel-Palestine War, or pandemic and the subsequent supply chain delays and disruptions because of those events. Find a current...
-
Primary Structure: Protein Structure: There are four levels of protein folding: primary, secondary, tertiary, and quaternary. The four levels are Amino acids represented in the diagram to the right....
-
What was your thoughts after watching the Khan video about deficits and debt? Did that help you understand the difference between the national debt and the deficit spending we engage in year after...

Study smarter with the SolutionInn App


