Consider the following four titrations (iiv): a. Rank the four titrations in order of increasing pH at
Question:
Consider the following four titrations (i–iv):
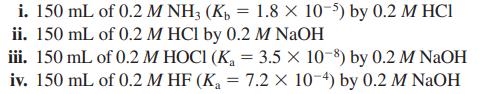
a. Rank the four titrations in order of increasing pH at the halfway point to equivalence (lowest to highest pH).
b. Rank the four titrations in order of increasing pH at the equivalence point.
c. Which titration requires the largest volume of titrant (HCl or NaOH) to reach the equivalence point?
Transcribed Image Text:
i. 150 mL of 0.2 M NH3 (K₂ = 1.8 × 10-5) by 0.2 M HCI ii. 150 mL of 0.2 M HCl by 0.2 M NaOH iii. 150 mL of 0.2 M HOCI (K₁ = 3.5 x 10-8) by 0.2 M NaOH iv. 150 mL of 0.2 MHF (K₁ = 7.2 Xx 10-4) by 0.2 M NaOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Rank the four titrations in order of increasing pH at the halfway point to equivalence lowest to h...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Consider the following four titrations: i. 100.0 mL of 0.10 M HCl titrated with 0.10 M NaOH ii. 100.0 mL of 0.10 M NaOH titrated with 0.10 M HCl iii. 100.0 mL of 0.10 M CH3NH2 titrated with 0.10 M...
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
The following are the Ledger Balance (in thousands) extracted from the books of Vaishnavi Bank Ltd as on March 31, 2016. The bank's Profit and Loss Account for the year ended and Balance Sheet as at...
-
Paul and Julie Leonards two-story home in Pascagoula, Mississippi, is only twelve feet above sea level and less than two hundred yards from the Gulf of Mexico. In 1989, the Leonards bought a...
-
A strand of wire has resistance 5.60. Find the net resistance of 120 such strands if they are. (a) Placed side by side to form a cable of the same length as a single strand, and (b) connected end to...
-
The John Gore Organization owns and operates the Charles Playhouse, a theater in Boston, Massachusetts. Evelyn Castillo has diabetes, a disability under the Americans with Disabilities Act (ADA)....
-
Two Indiana state senate candidates must decide which city to visit the day before the November election. The same four citiesIndianapolis, Evansville, Fort Wayne, and South Bendare available for...
-
4. Write as a single power, then evaluate. Express answers in rational form. a) V55 -16 b) c) 284 7 18 (9) d) 2
-
8. Jack Whitcombe and Sons is a consultant engineering firm. The accounts for the firm are as follows. Bank Car Expense HST Recoverable HST Payable J. Whitcombe, Capital J. Whitcombe, Drawings Fees...
-
To what reaction does the solubility product constant, Ksp, refer? Table 15.1 lists K sp values for several ionic solids. For any of these ionic compounds, you should be able to calculate the...
-
Calculate the value of the equilibrium constant for each of the following reactions in aqueous solution. a. HCHO + OHCHO + HO b. C,H,O, +H*
-
Graph the function. Give the domain and range. (x) = 2 x+2 - 4
-
10:00 Module name: Taxation Planning 2A Module code: TXP02A2 Year: 2022 Semester: First Assessment: Continuous Assessment 3 Release date: 1 April 2022 Submission deadline: 22 April 2022 INFORMATION:...
-
1. What could be the reasons for the price increase in offline retail stores? 2. From the information given in the case, can one conclude that there was a differential price response from the online...
-
On July 1, a company sells inventory on account to a customer for $40,000, with terms of 1/15, n/30. The company uses the net method to account for sales discounts. The customer pays the amount due...
-
What is one way that Netflix has changed the film/television industry? Can social media (Facebook, Twitter, Instagram, etc.) affect what movie/TV/song we might watch or listen to? Why or why not?...
-
A firm's end-of-year free cash flow is anticipated to be $14 million. The company's free cash flows are expected to grow 15 percent a year forever. The firm's weighted average cost of capital is 18...
-
Using Exhibit 12-9 as a guide, compare C&C Sports performance to the industry averages. What particular observations and recommendations do you have for George Douglas, president of C&CSports? 2011...
-
Gopher, Inc. developing its upcoming budgeted Costs of Quality (COQ) with the following information: Expense Item Budget Raw Materials Inspection $ 15,000 EPA Fine 200,000 Design Engineering 15,000...
-
The malonic ester synthesis cannot be used to make 2,2-dimethylhexanoic acid. Explain why not.
-
When a malonic ester synthesis is performed using excess base and 1,4-dibromobutane as the alkyl halide, an intramolecular reaction occurs, and the product contains a ring. Draw the product of this...
-
Starting with ethyl acetoacetate and using any other reagents of your choice, propose an efficient synthesis for each of the following compounds. (a) (b) (c) OH
-
Since dolphins cannot breathe underwater, they must reach the surface for air. The time dolphins can stay under the water surface depends on how deep they go and what kind of activities they engage...
-
Suppose all seven of the cubes from the center each face to the opposite face of a large 3 times 3 cube are removed and the resulting figure is dipped in red paint. How many cubes have red paint on 1...
-
There are two major tests of readiness for college, the ACT andthe SAT. ACT scores are reported on a scale from 1 to 36. The distribution of ACT scores isapproximately Normal with mean ? = 21.5 and...

Study smarter with the SolutionInn App


