How is acid strength related to the value of Ka? What is the difference between strong acids
Question:
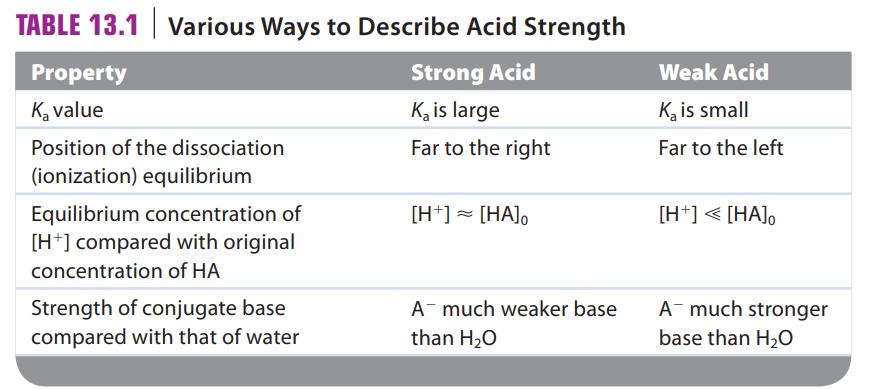
How is acid strength related to the value of Ka? What is the difference between strong acids and weak acids (see Table 13.1)? As the strength of an acid increases, what happens to the strength of the conjugate base? How is base strength related to the value of Kb? As the strength of a base increases, what happens to the strength of the conjugate acid?
Transcribed Image Text:
TABLE 13.1 Various Ways to Describe Acid Strength Strong Acid K₂ is large Far to the right Property K₂ value Position of the dissociation (ionization) equilibrium Equilibrium concentration of [H+] compared with original concentration of HA Strength of conjugate base compared with that of water [H+] = [HA]。 A much weaker base than H₂O Weak Acid K₂ is small Far to the left [H+] < [HA], A much stronger base than H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a The acid dissociation constant Ka is a measure of how strongly an acid dissociates in waterThe hig...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Chemists know that nitric and sulfuric acids are strong acids and that acetic acid is a weak acid. They would also agree that ethanol is at best a very weak acid. Acid strength is given directly by...
-
Draw a class diagram that reflect the following Java code segment as shown in Figure 4. public abstract class Vehicle { private int numberofWheels; public Vehicle () { this (0); } protected Vehicle...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Match each of the key terms with the definition that best fits it. ________ The process of ensuring that only authorized changes are made to a system. Here are the key terms from the chapter. The...
-
What would the order of inheritance have been if Ramish had died intestate? In June 2007, Bernard Ramish set up a $48,000 trust fund through West Plains Credit Union to provide tuition for his...
-
A rectangular coil of wire, 22.0 cm by 35.0 cm and carrying a current of 1.40 A, is oriented with the plane of its loop perpendicular to a uniform 1.50-T magnetic field, as shown in Fig. (a)...
-
Draw a cash flow diagram of any investment that exhibits both of the following properties: 1. The investment has a 4-year life. 2. The investment has a 10 percent/year internal rate of return.
-
The management of the Executive Furniture Corporation decided to expand the production capacity at its Des Moines factory and to cut back production at its other factories. It also recognizes a...
-
8) You own San Antonio's top rated food truck that sells the best carne asada tacos (good A) but the burger food truck (good B) next door just started a promotional sale: The burger truck...
-
S. L. P. Craft would like your help in developing a layout for a new outpatient clinic to be built in California. From analysis of another recently built clinic, she obtains the data shown in the...
-
Define or illustrate the meaning of the following terms: a. Amphoteric b. K w reaction c. K w equilibrium constant d. pH e. pOH f. pK w Give the conditions for a neutral aqueous solution at 25C, in...
-
At 35C, K = 1.6 10 -5 for the reaction If 2.0 moles of NO and 1.0 mole of Cl 2 are placed into a 1.0-L flask, calculate the equilibrium concentrations of all species. 2NOCI(g)2NO(g) + Cl(g)
-
A student using Appendix A2 wants to find their t-observed value for a dataset with 113 degrees of freedom. Unfortunately, there is no row in the table displaying the z-observed value for 113 degrees...
-
Use your MISSION AND VISSION to assess/ identify the external factors to be considered. To achieve both my goal and vision as a person, I must first have a crystal-clear understanding of both. When I...
-
The following are examples of best practice in financial management, for each, provide an example of following these best practices in the real estate industry. Do not use personal accounts
-
18. What, in the firm's opinion, are the greatest risks for financial integrity and internal control for real estate sector companies and developers?
-
Silly Filly Ltd is a recently established company specializing in the manufacture of talking toy horses for children. The Silly Filly range currently comprises three key products all of which are...
-
summarize chapter of the book Excel Chapter 1: Introduction to Excel. Introduction to Spreadsheets Mathematics and Formulas Workbook and Worksheet Enhancements page setup and printing
-
Bill Zimmerman is evaluating two new business opportunities. Each of the opportunities shown below has a ten-year life. Bill uses a 10% discount rate. Required a. Calculate the net present value of...
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
Draw the major product that is expected when each of the following compounds is treated with excess methyl iodide followed by aqueous silver oxide and heat: (a) Cyclohexylamine (b)...
-
Propose a synthesis for the following transformation (be sure to count the carbon atoms): Br
-
Compound A is an amine that does not possess a chirality center. Compound A was treated with excess methyl iodide and then heated in the presence of aqueous silver oxide to produce an alkene. The...
-
What is meant by Response? Give three (3) examples of an Emergency Management Response Instructions: 1. Write an essay on each of your examples. 2. provide a reference page at the end Citing your...
-
Discuss how this method of approaching a difficult situation may help you to overcome it. https://www.youtube.com/watch?v=s-ZKkDpeleE
-
Identify and detail the top 5-10 resources that Amazon would use to reduce and eliminate returns while ensuring that it is done in a sustainable manner (e.g., hiring a consultant, procuring necessary...

Study smarter with the SolutionInn App


