Without using Fig. 3.4, predict which bond in each of the following groups will be the most
Question:
Without using Fig. 3.4, predict which bond in each of the following groups will be the most polar.
Fig. 3.4
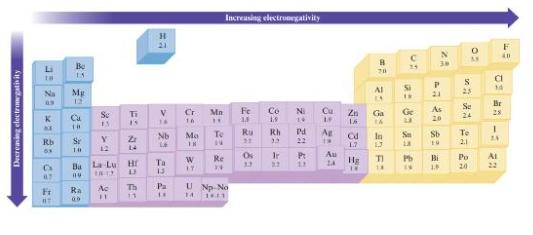
a. C-H, Si-H, Sn-H
b. Al-Br, Ga-Br, In-Br, Tl-Br
c. C-O or Si-O
d. O-F or O-CI
Transcribed Image Text:
Decreasing electronegativity Li Na * KE Rb 63 C 67 Fi #T Be 15 7 * =2 E Mg Se Y 12 Se 13 Ra 00 Ti 15 Ba La Lu Hr Bu Ac 11 Z 14 O D Th 11 H 21 V 14 Nb 23 22 4: Ta Pa Cr 16 9: Mo 18 W 17 Mn H Increasing electronegativity To 14 Re 14 U Np No 14 16.11 Fe 2= 2 82 Ru 3.3 Co 19 Rh 33 N Cu 2: 23 42 Pd 22 8: 2:33 1.3 Ag 1r P: Au 3.2 39 89 22 C 25 20 Al 18 D Ga Ge 23 In Sa 13 Zn 16. 1.7 Hg ROBS an Rd TI 14 IN a £: Pb N 30 P 21 As 20 Sb 19 Bi 1.8 0 S 23 To 2.1 AV Se 24 Po 20 a 14 Br 28 -: F 40 24 Ai
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a CH SiH SnH Carbon is more electronegative than silicon and ...View the full answer

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
Without using Fig. 13.3, predict which bond in each of the following groups is the most polar. a. COF, SiOF, GeOF b. POCl, SOCl c. SOF, SOCl, SOBr d. TiOCl, SiOCl, GeOCl e. COH, SiOH, SnOH f. AlOBr,...
-
Without using Fig. 3.4, predict which bond in each of the fol- lowing groups will be the most polar. Data in Fig. 3.4 a. C-F, Si-F, Ge-F b. P-Cl or S-Cl c. S-F, S-Cl, S-Br d. Ti-Cl, Si-Cl, Ge-Cl...
-
Do you have convincing evidence of sufficient computer skills to engage in online discussion forums, access online library resources, engage in online videoconferencing, and utilize word processing,...
-
You have been asked to evaluate possible sites for an Asian production facility that will manufacture your firms products and sell them to the Asian market. What real exchange rate considerations...
-
For a poll of voters regarding a referendum calling for a national value-added tax, design a sampling method to obtain the individuals in the sample. Be sure to support your choice. Which sampling...
-
Diageo North America, Inc., the owner of the Bulleit brand family of whiskeys, sued competitor W.J. Deutsch & Sons Ltd. for allegedly infringing upon the trade dress of Diageos Bulleit bottles....
-
Presented below is information for Yu Co. for the month of January 2012. Cost of goods sold ...... $212,000 Rent expense ........ $32,000 Freight-out .......... 7,000 Sales discounts ......... 8,000...
-
How do taxes and regulation impact the equity of budgeting decisions and as a public manager/administrator/analyst, how would one balance the need for taxation and the mandate to provide equitable...
-
Which of the following incorrectly shows the bond polarity? Show the correct bond polarity for those that are incorrect.
-
Describe the type of bonding that exists in the Cl 2 (g) molecule. How does this type of bonding differ from that found in the HCl(g) molecule? How is it similar?
-
In a survey of 420 U.S. females ages 18 to 64, 279 say they have gone to the dentist in the past year. Construct 90% and 95% confidence intervals for the population proportion. Interpret the results...
-
5. Ogden Electronics (OE) produced 60,000 widgets last month. OE started the month with $100,000 worth of inventory in Finished Goods. The company incurred $140,000 of various utility and rent...
-
95. Ferguson Co. incurs $568,000 in fixed costs while producing three products with the following characteristics: Sales Mix Unit Contribution Contribution Product (Units) Margin Margin Ratio TOR 5...
-
Bagwell Corporation is considering the purchase of a small fleet of trucks for its delivery operation. The invoice price of the trucks is $220,000; sales tax on the purchase is $15,000; shipping and...
-
You are the owner of the only concrete producer in your vicinity. You are able to charge $110 per cubic yard and produce 60,000 cubic yards per year, thereby making an economic profit of $300,000 per...
-
In January 2019, Triton building had 1800 units with a vacancy rate of 5%. Over the following 3 years they built 300 additional units. In January 2022 the total units were 2100 and the vacancy rate...
-
Weida Surveying, Inc., provides land surveying services. During September, its transactions included the following: Sept. 1 Paid rent for the month of September, $4,400. Sept. 3 Billed Fine Line...
-
The comparative statements of financial position of Menachem NV at the beginning and end of the year 2019 appear below. Net income of ¬34,000 was reported, and dividends of ¬23,000 were paid...
-
The concentration of Pb 2+ in a solution saturated with PbBr 2 (s) is 2.14 10 -2 M. Calculate K sp for PbBr 2 .
-
Approximately 0.14 g nickel(II) hydroxide, Ni(OH) 2 (s), dissolves per liter of water at 20 C. Calculate K sp for Ni(OH) 2 (s) at this temperature.
-
Write balanced equations for the dissolution reactions and the corresponding solubility product expressions for each of the following solids. a. Ag 2 CO 3 b. Ce(IO 3 ) 3 c. BaF 2
-
You set up a college fund in which you pay $3200 each year at the beginning of the year. How much money (in $) will you have accumulated in the fund after 24 years, if your fund earns 8.5% compounded...
-
Cooper wants to retire with $720000 in his retirement account exactly 38 years from today. He will make annual deposits at the end of each year to fund his retirement account. If he can earn 7...
-
Dawgpound Incorporated has a bond trading on the secondary market that will mature in four years. The bond pays an annual coupon with a coupon rate of 9.25%. Dawgpound bonds currently trade at...

Study smarter with the SolutionInn App


