Bromine-containing species play a role in environmental chemistry. For example, they are evolved in volcanic eruptions. (a)
Question:
Bromine-containing species play a role in environmental chemistry. For example, they are evolved in volcanic eruptions.
(a) The following molecules are important in bromine environmental chemistry: HBr, BrO, and HOBr. Which is an odd-electron molecule?
(b) Use bond dissociation enthalpies to estimate ΔrH for three reactions of bromine: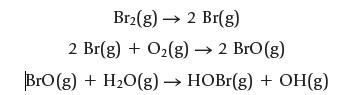
(c) Using bond dissociation enthalpies, estimate the standard enthalpy of formation of HOBr(g) from H2(g), O2(g), and Br2(g).
(d) Are the reactions in parts (b) and (c) exothermic or endothermic?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





