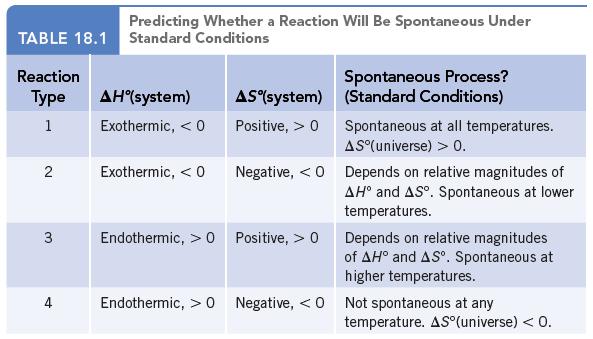
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1.
Question:
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1.![]()
Δr H° = −673 kJ/mol-rxn
Δr S° = 60.4 J/K · mol-rxn![]()
Δr H° = 490.7 kJ/mol-rxn
Δr S° = 197.9 J/K · mol-rxn
Data given in Table 18.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





