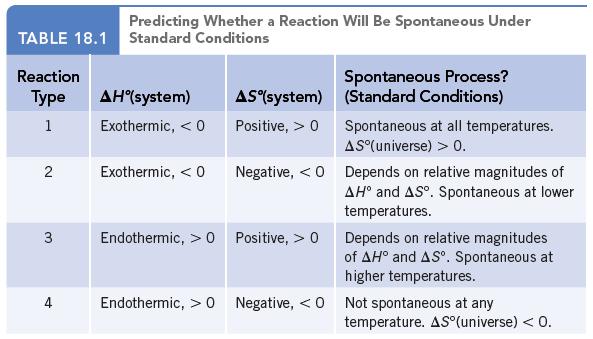
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1.
Question:
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1.![]()
Δr H° = −851.5 kJ/mol-rxn
Δr S° = −375.2 J/K · mol-rxn![]()
Δr H° = 66.2 kJ/mol-rxn
Δr S° = −121.6 J/K · mol-rxn
Data given in Table 18.1
Transcribed Image Text:
(a) Fe₂O3(s) + 2 Al(s) → 2 Fe(s) + Al₂O3(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Since this reaction has enthalpy change and entropy change negative When bot...View the full answer

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
MARGINAL COST EQUATION The marginal cost formula is nothing but the mathematical representation to capture the incremental cost impact due to the production of additional units of a good or service....
-
Classify each of the reactions according to one of the four reaction types summarized in Table 18.1. r H = 673 kJ/mol-rxn r S = 60.4 J/K mol-rxn r H = 490.7 kJ/mol-rxn r S = 197.9 J/K mol-rxn...
-
Classify each of the following reactions as one of the four possible types summarized in Table 19.3: (a) (b) (c) N2(g) 3 F2(g)2NF3(g) AH249 kJ; AS278 J/K N2(g) + 3C12(g) --> 2NC3(g) AH 460 kJ; AS...
-
Suppose you have the following training set, and fit a logistic regression classifier : ho(x) = g(00+011+0x2) O O O Which of the following are true? Check all that apply. a) Adding polynomial...
-
Critically review Mo Chengs approach to sampling and her subsequent data collection strategy. Can Mo Cheng meet her stated objective? Im doing really well Mum, you dont have to worry about me. Ive...
-
Select a value of \(C\) in Figure P10-3 3 so that \(V_{\mathrm{O}}(s) /\) \(V_{\mathrm{S}}(s)\) has a natural pole at \(s=-10 \mathrm{Mrad} / \mathrm{s}\).
-
The processes in the Carnot cycle are carried out in a/an (a) Reversible fashion (b) Irreversible fashion (c) Neither (a) nor (b) (d) Both (a) and (b).
-
Salaur Company is evaluating a lease arrangement being offered by TSP Company for use of a computer system. The lease is noncancelable, and in no case does Salaur receive title to the computers...
-
How does remote work or virtual collaboration affect employees' sense of connection and commitment to an organization ?
-
Is the reaction Si(s) + 2 Cl 2 (g) SiCl 4 (g) spontaneous under standard conditions at 298.15 K? Answer this question by calculating S(system), S(surroundings), and S(universe). (Define reactants...
-
Calculate the standard entropy change for the following reactions at 25C. Comment on the sign of r S. (a) 2 Na(s) + 2 H 2 O() 2 NaOH(aq) + H 2 (g) (b) Na 2 CO 3 (s) + 2 HCl(aq) 2 NaCl(aq) + H 2...
-
Every IPO is unique, but what are the basic empirical regularities in IPOs?
-
Suppose Carol's stock price is currently $20 and does not currently pay dividends. In the next 6 months it will either fall to $10 or rise to $40. What is the current value of a European 6-month call...
-
Workplace safety has economic, reputational, and ethical implications. What do you think these implications may be? what are the best aspects in employment law in the workplace?
-
Half a liter of gas in an air conditioner is initially at a temperature of 25 degrees Celsius and 2.0 atm of pressure. The volume of the gas is rapidly expanded to 2 L and the pressure of the gas...
-
A 1.3 kg book is lying on a 0.75 m -high table. You pick it up and place it on a bookshelf 2.3 m above the floor. During this process, how much work does gravity do on the book? During this process,...
-
Coronado, Inc., manufactures clamps used in the overhead bin latches of several leading airplane models. Brian Lee, president of Coronado, Inc., has gathered the following cost information from the...
-
What are special assessments? How do governments account for them?
-
r = 0.18 Find the coefficients of determination and non-determination and explain the meaning of each.
-
Why is the magnitude of the electron affinity for a given element smaller than the magnitude of the first ionization energy? Na Ne Li Be Element First Ionization N 13.6 24.6 5.4 13.6 9.3 8.3 14.5...
-
Calculate the position of the maximum in the radial distribution function for Li 2+ in its ground state using the wave function in P21.13.
-
The ground-state wave function of Li 2+ is -1/2 (Z/a 0 ) 3/2 e -Zr /a 0 , where Z is the nuclear charge. Calculate the expectation value of the potential energy for Li 2+ .
-
Apollo Data Systems is considering a promotional campaign that will increase annual credit sales by $684,000. The company has a 60% cost of goods sold and will require investments in accounts...
-
Schylar Pharmaceuticals, Inc., plans to sell 115,000 units of antibiotic at an average price of $23 each in the coming year. Total variable costs equal $846,400. Total fixed costs equal $7,400,000....
-
Cullumber Manufacturing Company has four operating divisions. During the first quarter of 2022, the company reported aggregate income from operations of $187,700 and the following divisional results:...

Study smarter with the SolutionInn App


