Maleic anhydride, C 4 H 2 O 3 , can be produced by the oxidation of benzene
Question:
Maleic anhydride, C4H2O3, can be produced by the oxidation of benzene (Study Question 141). It can also be produced from the oxidation of butene.
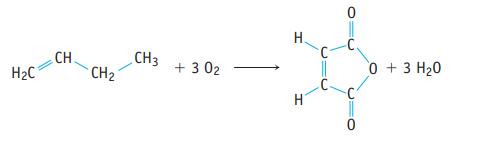
(a) What is the % atom economy for the synthesis of maleic anhydride from butene by this reaction?
(b) If 1.02 kg of maleic anhydride is produced from exactly 1.00 kg of butene, what is the percent yield of the anhydride? What mass of the by-product H2O is also produced?
Data given in Question 141
Benzene, C6H6, is a common compound, and it can be oxidized to give maleic anhydride, C4H2O3, which is used in turn to make other important compounds.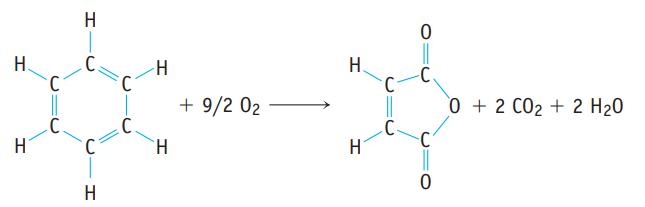
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





