Phosphoric acid can supply one, two, or three H 3 O+ ions in aqueous solution. Write balanced
Question:
Phosphoric acid can supply one, two, or three H3O+ ions in aqueous solution. Write balanced equations (like those for sulfuric acid on page 142) to show this successive loss of hydrogen ions.
Data given in page 142
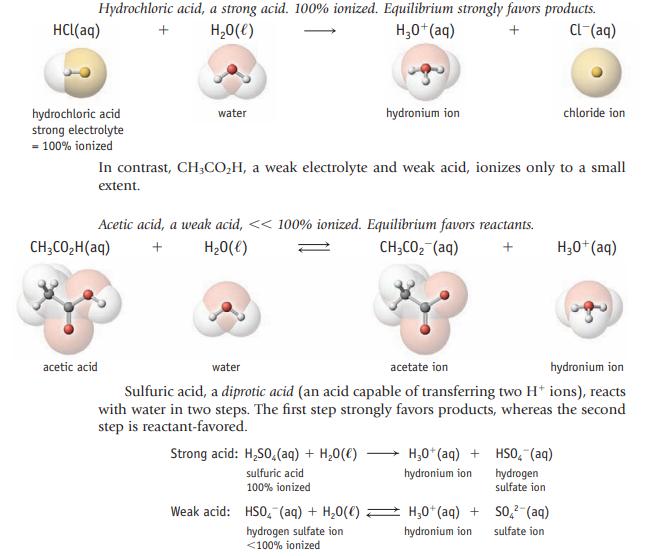
Transcribed Image Text:
Hydrochloric acid, a strong acid. 100% ionized. Equilibrium strongly favors products. + H₂O(l) H₂0+ (aq) + CL-(aq) HCl(aq) hydrochloric acid strong electrolyte = 100% ionized water CH3CO₂H(aq) acetic acid In contrast, CH3CO₂H, a weak electrolyte and weak acid, ionizes only to a small extent. Acetic acid, a weak acid, << 100% ionized. Equilibrium favors reactants. + H₂O(l) CH3CO₂ (aq) + hydronium ion Strong acid: H₂SO4 (aq) + H₂O(l) sulfuric acid 100% ionized Weak acid: HSO (aq) + H₂O(C) hydrogen sulfate ion <100% ionized water acetate ion hydronium ion Sulfuric acid, a diprotic acid (an acid capable of transferring two H+ ions), reacts with water in two steps. The first step strongly favors products, whereas the second step is reactant-favored. H₂0+ (aq) + hydronium ion chloride ion HSO₂ (aq) hydrogen sulfate ion H₂0+ (aq) + SO² (aq) hydronium ion sulfate ion H3O+(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
Phosphoric acid H3PO4 is a triprotic acid meaning it can rele...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Oxalic acid, H 2 C 2 O 4 , which is found in certain plants, can provide two hydronium ions in water. Write balanced equations (like those for sulfuric acid on page 142) to show how oxalic acid can...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
Peroxodisulfate ions, S 2 O 8 2 , react with iodide ions in aqueous solution to form iodine and sulfate ions. S 2 O 8 2 (aq) + 2I (aq) 2SO 4 2 (aq) + I 2 (aq) equation 1 The initial rates of...
-
Data for Sabanci Holding can be found in the table below. Theincome statement items correspond to revenues or costs during theyear ending in either 2018 or 2019. The balance sheet itemscorrespond t 2...
-
We stated in this chapter that GNP accounts avoid double counting by including only the value of final goods and services sold on the market. Should the measure of imports used in the GNP accounts...
-
There are several extensions of linear regression that apply to exponential growth and power law models. Problems 2225 will outline some of these extensions. First of all, recall that a variable...
-
In which engine values are used? (a) Two-stroke engine (b) Four-stroke engine (c) In both engines (d) None of the above
-
Clear channel, an owner of multiple radio stations with the Top 40 format, recently bought rock concert promoter Live Nation. How would this affect prices for concert tickets or rates for radio...
-
You have been meeting on a team for the past couple of weeks, and while you really get along well with your teammates, it is clear that very little "work" is getting done at these meetings. The other...
-
Write a balanced equation for the ionization of nitric acid in water.
-
Predict the products of each precipitation reaction. Balance the equation, and then write the net ionic equation. (a) Pb(NO3)2(aq) (b) Ca(NO3)2(aq) (c) Ca(NO3)2(aq) + KBr(aq) + KF(aq) + NaCO4 (aq)
-
Daisy takes her roommate's credit card, intending to charge expenses that she incurs on a vacation. Her first stop is a gas station, where she uses the card to pay for gas. With respect to the gas...
-
Accounts receivable is $ 6 , 0 7 6 in 2 0 2 0 and $ 5 , 6 2 4 in 2 0 2 1 The acquisition cost of plant, property and equipment is $ 2 0 , 5 8 0 in 2 0 2 0 and $ 2 6 , 4 0 4 in 2 0 2 1 Accumulated...
-
Paige's Oasis Supplies, a backyard and garden super-store, has retained an accountant to assess the variances in their budget for more efficient ordering and sale practices. The accountant compiled...
-
Mike is paid to advise his clients on how to invest their money. One day, he is reviewing the financial statements for a publicly traded company, and believes the company is poised to gain...
-
Consider a 10 year bond with face value $1,000, pays 6% coupon annually and has a yield-to-maturity of 7%. How much would the approximate percentage change in the price of bond if interest rate in...
-
In a standard air wedge interference experiment, calculate the thickness (in micrometres) of the wedge which gives the 10th bright fringe for light of wavelength 479 nm. Give your answer to 2d.p.
-
On January 1, 2011, Peres Company purchases 80% of the common stock of Soap Company for $308,000. On this date, Soap has common stock, other paid-in capital in excess of par, and retained earnings of...
-
Prepare a stock card using the following information A company is registered for GST which it pays quarterly, assume GST was last paid on the 30th of June 2019. It uses weighted average cost...
-
Choose a Grignard reagent and a ketone that can be used to produce each of the following compounds: (a) 3-methyl-3-pentanol (b) 1-ethylcyclohexanol (c) Triphenylmethanol (d) 5-phenyl-5-nonanol
-
You are working in a laboratory, and you are given the task of converting cyclopentene into 1,5 pentanediol. Your first thought is simply to perform an ozonolysis followed by reduction with LAH, but...
-
Predict the major product(s) from the treatment of acetone with the following compounds: (a) [H + ], NH 3 , (H 2 O) (b) [H + ], CH 3 NH 2 , (H 2 O) (c) [H + ], excess EtOH, (H 2 O) (d)[H + ], (CH 3 )...
-
What are three resource management tools that facilitate family decision making? Discuss how to structure a successful performance appraisal interview?
-
You are provided with the following information for Carla Vista Co., effective as of its April 30, 2025, year-end. Accounts payable $844 Accounts receivable 840 Accumulated depreciation-equipment 640...
-
How can conflict theory proposed a solution for crime and education in a community? Provide references!

Study smarter with the SolutionInn App


