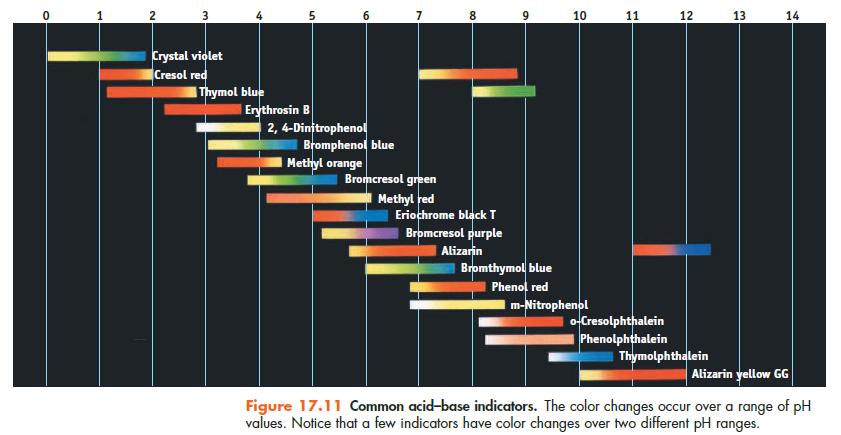
Using Figure 17.11, suggest an indicator to use in each of the following titrations: (a) The weak
Question:
Using Figure 17.11, suggest an indicator to use in each of the following titrations:
(a) The weak base pyridine is titrated with HCl.
(b) Formic acid is titrated with NaOH.
(c) Ethylenediamine, a weak diprotic base, is titrated with HCl.
Data given in Figure 17.11
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





