Using standard enthalpies of formation, verify that 2680 kJ of energy is released in combustion of 100.0
Question:
Using standard enthalpies of formation, verify that 2680 kJ of energy is released in combustion of 100.0 g of ethanol. 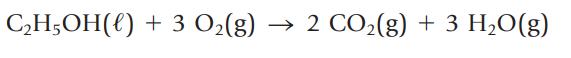
Transcribed Image Text:
C₂H5OH() + 3 O₂(g) 2 CO₂(g) + 3 H₂O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
To verify that 2680 kJ of energy is released in the combustion of 1000 g of ethanol using standard ...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Look up the standard enthalpies of formation of glucose and ethanol (Table 9.8), and verify that eq. (26.4) is exothermic. Then verify that the gravimetric energy density (relative to combustion) of...
-
Calculate the standard enthalpies of formation of (a) KClO 3 (s) from the enthalpy of formation of KCl, (b) NaHCO 3 (s) from the enthalpies of formation of CO 2 and NaOH together with the following...
-
There are many ethical and legal obligations that agents must follow when delivering a real estate service - whether sales, property management or marketing. Select the ethical and legal obligations...
-
For each of these utility functions, find the optimal consumption choices z and y for a consumer with budget w = 1, who faces prices pz = 0.05 and Py = 0.3. Are the goods substitutes or complements,...
-
Genuine Reproductions (GR) plans on increasing next years sales by 20 percent while maintaining its same average inventory in dollars of $250,000. (a) Calculate the expected inventory turnover for...
-
On the morning of December 6, you purchased a futures contract for one eur at a rate of inr/eur 55. The following table gives the subsequent settlement prices and the p.a. bid-ask interest rates on a...
-
In the region just downstream of a sluice gate, the water may develop a reverse flow region as is indicated in Fig. P4.59 and Video V10.9. The velocity profile is assumed to consist of two uniform...
-
The following selected information was extracted from the 20x1 accounting records of Surgical Products, Inc.: Raw-material-purchases ..............$350,000 Direct labor .................. 508,000...
-
List HOW each of the 5 main adult learning methods can be each used in a T&D program to help build organizational development of that competency.
-
According to the Nutrient Data Laboratory website (www.ars.usda.gov/ba/bhnrc/ndl), corn oil contains 3766 kJ of energy per 100. g serving. (a) What is the energy content of 100. g of corn oil in...
-
You drink 350 mL of diet soda that is at a temperature of 5C. (a) How much energy will your body expend to raise the temperature of this liquid to body temperature (37C)? Assume that the density and...
-
In words and symbols, write the two components of the rate of return on a stock investment for a holding period of one year.
-
The following cost data for the month of May were taken from the records of the Terrence Manufacturing Company: ( CIA adapted ) Depreciation on factory equipment $ 1 , 3 0 0 Depreciation on sales...
-
The following is the post-closing trial balance for the Whitlow Manufacturing Corporation as of December 31, 2023. Account Title 3 Cash Accounts receivable Inventory Equipment Accumulated...
-
2-dz zdz - Evaluate along the curve Y=x
-
Mock cash flow statement for Big Rock Candy Mountain Mining Co. for fiscal 2020. Assuming net income of 24875, depreciation expense of 28995, and change in inventory of -652, please fill in the total...
-
Recently we all saw the U.S. shoot down, off the coast of South Carolina, a Chinese balloon thought to be collecting sensitive data across the United States. Given the topic under discussion, Cyber...
-
How is the use of a balanced scorecard as a performance evaluation system helpful to companies?
-
Solve each equation or inequality. |6x8-4 = 0
-
Hydroxide is not a suitable base for deprotonating acetylene: Explain why not. Can you propose a base that would be suitable? - -: : - Acetylene Hydroxide
-
Amino acids, such as glycine, are the key building blocks of proteins and will be discussed in greater detail in Chapter 25. At the pH of the stomach, glycine exists predominantly in aprotonated form...
-
In each compound below, two protons are clearly identified. Determine which of the two protons is more acidic. (a) (b) (c) (d) N' C-
-
A trend that has gained popularity in recent years is Bring-Your-Own-Device (BYOD). Rather than dictate the type of device that employees should use, a company may provide a set amount of money...
-
Explain the reason we arrange the cable as per instructions. Explain the purpose of each pin on the RJ45 modular plug. How many various versions of wiring scheme exist for Ethernet cables? What is...
-
You are responsible for providing IT support for Seneca College teaches and students. A professor contacts you and asked for help. It seems none of his students can connect to his on-line class....

Study smarter with the SolutionInn App


