Calculate the potential for each of the voltaic cells in Exercise 18.43 when the concentrations of the
Question:
Calculate the potential for each of the voltaic cells in Exercise 18.43 when the concentrations of the soluble species and gas pressures are as follows:![(a) [Cu+] = 0.050 M, [Ni+] = 1.40 M (b) Pci 320 torr (c) [I]= 0.0010 M, Pcl = 0.300 atm, [C1-] = 0.60 M](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/7/1/1/324659bd49cd81c81704711324422.jpg)
Exercise 18.43
Use the standard reduction potentials in Table18.1 to find
(a) A metal ion that reduces Ni2+.
(b) A metal ion that can oxidize Cu.
(c) A metal ion that is reduced by Cr2+ but not H2.
Table 18.1 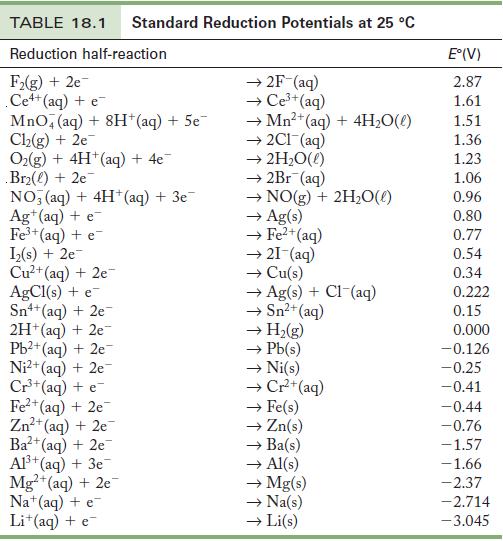
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





