Using Table 9.4, calculate the energy required to break all of the bonds in one mole of
Question:
Using Table 9.4, calculate the energy required to break all of the bonds in one mole of the following compounds.
(a) CH2CF2
(b) N2H4
Table 9.4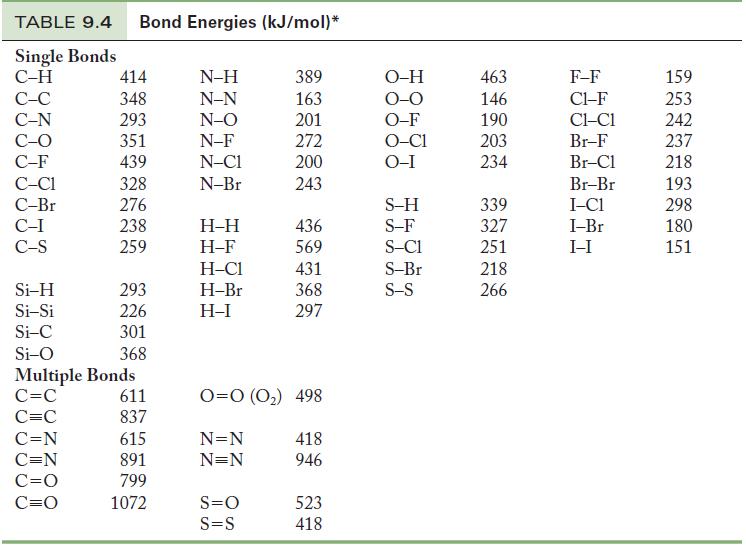
Transcribed Image Text:
TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H C-C C-N C-O C-F C-C1 C-Br C-I C-S Si-H Si-Si Si-C Si-O 414 348 293 351 439 328 276 238 259 C=N C=N C=O C=O 293 226 301 368 Multiple Bonds C=C C=C 611 837 615 891 799 1072 N-H N-N N-O N-F N-C1 N-Br H-H H-F H-C1 H-Br H-I 389 163 201 272 200 243 S=O S=S 436 569 431 368 297 0=0 (0₂) 498 N=N N=N 418 946 523 418 O-H O-O O-F O-C1 O-I S-H S-F S-C1 S-Br S-S 463 146 190 203 234 339 327 251 218 266 F-F C1-F CLC1 Br-F Br-Cl Br-Br I-C1 I-Br I-I 159 253 242 237 218 193 298 180 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a dissociation bonds broken EAH bonds formed diss...View the full answer

Answered By

Shebla K
I am an MBA graduate having experience as an Assistant Professor at University level for two years. I always prepare well for a class as I believe that only if you become an ocean you can give a bucket of water. Being a teacher was not only my profession but also my passion.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Using Table 9.4, calculate the energy required to break all of the bonds in one mole of the following compounds. (a) NH 3 (b) CH 3 OH Table 9.4 TABLE 9.4 Bond Energies (kJ/mol)* Single Bonds C-H...
-
The specific heat capacity of silver is 0.24 JoC-1g-1. a. Calculate the energy required to raise the temperature of 150.0 g Ag from 273 K to 298 K. b. Calculate the energy required to raise the...
-
Calculate the energy required to assemble the array of charges shown in Figure P25.34, where a = 0.200 m, b = 0.400 m, and q = 6.00 +C.
-
Describe An Analysis of the relationship between Employee Engagement, Satisfaction, and Organizational Performance at Enterprise Mobility
-
Using atomic weight, crystal structure, and atomic radius data tabulated inside the front cover, compute the theoretical densities of lead, chromium, copper, and cobalt, and then compare these values...
-
Boeing Company has had its financial ups and downs. Recently, the CFO for Boeing helped turn its problems around by analyzing the amount of value each product was providing to the companys bottom...
-
For the Grunfeld example, replicate Tables 13.6, 13.7 and 13.8, i.e., (i) obtain the Breusch and Pagan test based on the fixed effects residuals using Stata's command xttest2. (ii) obtain Pesaran's...
-
At the beginning of its operations in March 2016, Mastiff Supplies Ltd. began with 7,500 units of inventory that it purchased at a cost of $7.00 each. The companys purchases during March were as...
-
at the beginning of the year addisons companys assests are 1 5 8 0 0 0 and its equity is 1 1 8 5 0 0 . during the year assets increase 8 0 0 0 0 and liabilities increase 4 8 0 0 0 . what is the...
-
Which molecule has the most polar bond: N 2 , BrF, or ClF? Use an arrow to show the direction of polarity in each bond.
-
Using Table 9.4, calculate an approximate enthalpy change for (a) The reaction of molecular hydrogen (H 2 ) and molecular oxygen (O 2 ) in the gas phase to produce 2 mol water vapor. (b) The reaction...
-
Pegged orders are created to follow the NBBO as it moves. In addition to the installation of the speed bump, the IEX has provided the primary peg order, which is intended to protect small or retail...
-
6.10 If we integrate the Maxwell-Boltzmann distribution over from some momentum up, we are faced with error functions 00 2 erfc(y) = = 7/7 S, dr. d.x. Problems 87 [See Handbook of Mathematical...
-
4.4 The window of a Geiger-Muller detector is made of mica and has a thickness of 0.02 kg/m (p= 2.6 10 kg/m). For mica composition, use NaAl-Si-O0(OH). a. What is the minimum electron energy that...
-
To make a Venn diagram It should take up about half of one letter-sized sheet of paper. Fill in the Venn diagram with information about the bonds - ioinic bond and covalent bond . The left-hand...
-
What is the direction (angle (in degrees), measured counterclockwise relative to +x axis) of vector of magnitude Q? Please print your answer with 1 decimal place. P 0 1 1 X
-
A skier travels down the trail as shown below. The slope is inclined at an angle of 22 and the coefficient of kinetic friction is 0.2. a) Draw the free body diagram of the skier. [2 marks] b) Using...
-
True or False: 1. Activist economists believe that discretionary macroeconomic policy can make the economy less unstable. 2. Most economists think that unemployment will remain close to the natural...
-
You are interested in investing and are considering a portfolio comprised of the following two stocks. Their estimated returns under varying market conditions are provided: (note: it is difficult to...
-
A shaft is made of an aluminum alloy having an allowable shear stress of Ï allow = 100 MPa. If the diameter of the shaft is 100 mm, determine the maximum torque T that can be transmitted. What...
-
The solid shaft of radius r is subjected to a torque T. Determine the radius r' of the inner core of the shaft that resists one-quarter of the applied torque (T/4). Solve the problem two ways:...
-
The solid shaft of radius r is subjected to a torque T. Determine the radius r of the inner core of the shaft that resists one-half of the applied torque (T>2). Solve the problem two ways: (a)...
-
Consider the standard, built- in accessibility features on a computer or mobile device. When might a student benefit from a separate device or technology as opposed to using the standard...
-
What are some digital channels for communicating to a professional audience, and what are the advantages and disadvantages of each channel?
-
Provide an example of a situation that you have experienced or that you look out for as an IT professional, that could compromise the security of information contained on a Government website....

Study smarter with the SolutionInn App


