A membrane separates two fluids. One of the fluids is well mixed and the other is unmixed
Question:
A membrane separates two fluids. One of the fluids is well mixed and the other is unmixed as shown in Figure P5.36. On the side of the membrane adjacent to the unmixed fluid, an enzyme is attached that can react with substrate present in the fluids. The rate of reaction per unit surface area is given by:
\[-r_{s}=\frac{k_{s}^{\prime \prime} K_{s} c_{s}}{K_{i} K_{m}+K_{i} c_{s}}\]
where \(K_{m}\) is the Michaelis constant and \(K_{i}\) is an inhibition constant. Excess substrate at the membrane surface can diffuse through the membrane to the well-mixed side.
a. Derive the differential equation governing the concentration profiles in the unmixed fluid and in the membrane. Assume all interfacial resistances are one and that we have dilute solutions.
b. What are the boundary conditions for this problem?
c. Is it possible to have \(c_{s o}=c_{s i}\) yet still have a flux through the membrane from the unmixed to the well-mixed fluid? From the well-mixed to the unmixed fluid? Why?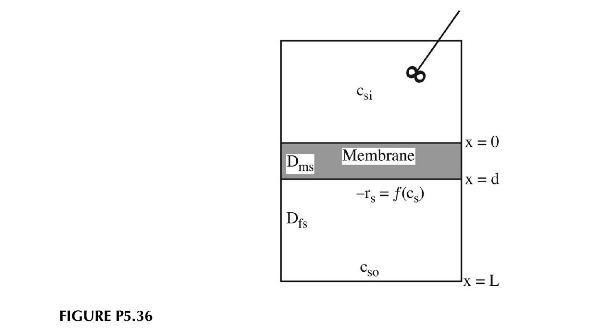
Step by Step Answer:






