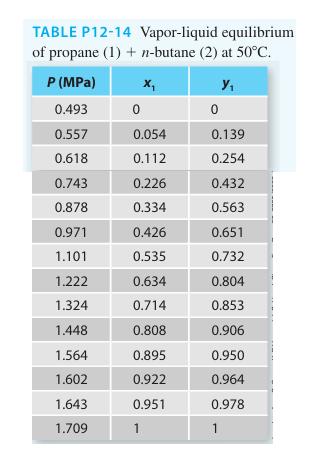
Consider the propane (1) + n-butane (2) system at 50C. Using a gamma-phi modeling approach, predict the
Question:
Consider the propane (1) + n-butane (2) system at 50°C. Using a gamma-phi modeling approach, predict the Pxy diagram for the system using the van Laar equation and the virial equation. Compare the predicted values with the experimental data provided (on the same plot) in Table P12-14. Also add the Raoult’s Law predictions and comment on the utility of the gamma-phi modeling approach for this system at this state.
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:




