Experimental data for the excess molar volume of 1-propanol (1) + 1-hexene (2) system at 298.15 K
Question:
Experimental data for the excess molar volume of 1-propanol (1) + 1-hexene (2) system at 298.15 K is provided in Table P9-29. If the pure component densities at 298.15 K are 0.79965 g/cm3 for 1-propanol and 0.66828 g/cm3 for 1-hexene, do the following.
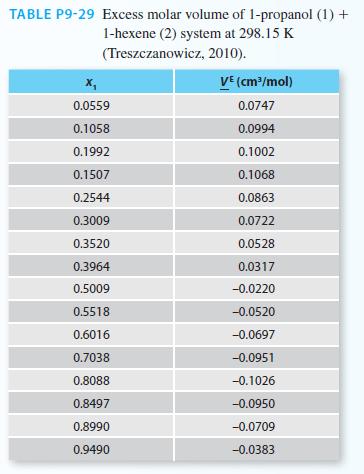
A. Plot the solution molar volume as a function of composition of 1-propanol.
B. Fit the solution molar volume to an appropriate functional form.
C. Determine the partial molar volume for both substances when the system is equimolar.
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:




